当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Intrinsic Effect of Carbon Supports on the Activity and Stability of Precious Metal Based Catalysts for Electrocatalytic Alcohol Oxidation in Fuel Cells: A Review.
ChemSusChem ( IF 8.4 ) Pub Date : 2020-02-18 , DOI: 10.1002/cssc.202000048 Jin Zhang 1 , Shanfu Lu 1 , Yan Xiang 1 , San Ping Jiang 2
ChemSusChem ( IF 8.4 ) Pub Date : 2020-02-18 , DOI: 10.1002/cssc.202000048 Jin Zhang 1 , Shanfu Lu 1 , Yan Xiang 1 , San Ping Jiang 2
Affiliation
|
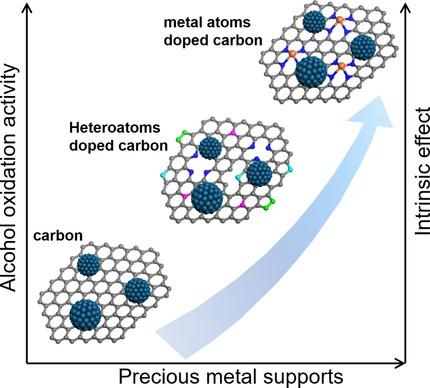
Electrocatalyst supports, in particular carbonaceous materials, play critical roles in the electrocatalytic activity and stability of precious metal group (PMG)-based catalysts such as Pt, Pd, and Au for the electrochemical alcohol oxidation reaction (AOR) of fuels such as methanol and ethanol in polymer electrolyte membrane fuel cells (PEMFCs). Carbonaceous supports such as high surface area carbon provide electronic contact throughout the catalyst layer, isolate PMG nanoparticles (NPs) to maintain high electrochemical surface area, and provide hydrophobic properties to avoid flooding of the catalyst layer by liquid water produced. Compared to high surface area carbon, PMG catalysts supported on 1D and 2D carbon materials such as graphene and carbon nanotubes show enhanced activity and durability due to the intrinsic effect of the underlying carbonaceous supports on the electronic states of PMG NPs. The modification of the electronic environment, in particular the d-band centers of PMG NPs, weakens the adsorption of AOR intermediates, facilitates breaking of the C-C bonds, and thus enhances the electrocatalytic activity of PMG catalysts. The doping of heteroatoms further facilitates the electrocatalytic activity for the AOR through the structural, bifunctional, and electronic effects, in addition to the enhanced dispersion of PMG NPs in the carbon support. The prospects for the development of effective PMG-based catalysts for high-performance alcohol-fuel-based PEMFCs is discussed.
中文翻译:

碳载体对贵金属基燃料在燃料电池中电催化醇氧化的活性和稳定性的内在影响:综述。
电催化剂载体,特别是碳质材料,在贵金属基(PMG)基催化剂(例如Pt,Pd和Au)的电催化活性和稳定性中,对于燃料(例如甲醇和甲醇)的乙醇氧化反应(AOR)起着关键作用。聚合物电解质膜燃料电池(PEMFC)中的乙醇。碳质载体(例如高表面积碳)在整个催化剂层中提供电子接触,隔离PMG纳米颗粒(NPs)以维持高电化学表面积,并提供疏水性,以避免所产生的液态水淹没催化剂层。与高表面积碳相比,一维和二维碳材料(例如石墨烯和碳纳米管)上承载的PMG催化剂由于基础碳质载体对PMG NPs电子态的内在影响而显示出增强的活性和耐久性。电子环境的改变,特别是PMG NPs的d波段中心,削弱了AOR中间体的吸附,促进了CC键的断裂,从而增强了PMG催化剂的电催化活性。除了增强PMG NPs在碳载体中的分散性之外,杂原子的掺杂还通过结构,双功能和电子效应进一步促进了AOR的电催化活性。讨论了开发用于高效醇燃料基PEMFC的有效PMG基催化剂的前景。
更新日期:2020-02-18
中文翻译:

碳载体对贵金属基燃料在燃料电池中电催化醇氧化的活性和稳定性的内在影响:综述。
电催化剂载体,特别是碳质材料,在贵金属基(PMG)基催化剂(例如Pt,Pd和Au)的电催化活性和稳定性中,对于燃料(例如甲醇和甲醇)的乙醇氧化反应(AOR)起着关键作用。聚合物电解质膜燃料电池(PEMFC)中的乙醇。碳质载体(例如高表面积碳)在整个催化剂层中提供电子接触,隔离PMG纳米颗粒(NPs)以维持高电化学表面积,并提供疏水性,以避免所产生的液态水淹没催化剂层。与高表面积碳相比,一维和二维碳材料(例如石墨烯和碳纳米管)上承载的PMG催化剂由于基础碳质载体对PMG NPs电子态的内在影响而显示出增强的活性和耐久性。电子环境的改变,特别是PMG NPs的d波段中心,削弱了AOR中间体的吸附,促进了CC键的断裂,从而增强了PMG催化剂的电催化活性。除了增强PMG NPs在碳载体中的分散性之外,杂原子的掺杂还通过结构,双功能和电子效应进一步促进了AOR的电催化活性。讨论了开发用于高效醇燃料基PEMFC的有效PMG基催化剂的前景。


























 京公网安备 11010802027423号
京公网安备 11010802027423号