当前位置:
X-MOL 学术
›
Eur. J. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fibroblast transdifferentiation promotes conversion of M1 macrophages and replenishment of cardiac resident macrophages following cardiac injury in mice.
European Journal of Immunology ( IF 5.4 ) Pub Date : 2020-02-18 , DOI: 10.1002/eji.201948414 Hongxiang Lu 1, 2 , Rong Chen 1, 2 , Prince Amoah Barnie 1 , Yu Tian 1, 2 , Shiqing Zhang 1, 2 , Huaxi Xu 2 , Subrata Chakrabarti 3 , Zhaoliang Su 1, 2, 4
European Journal of Immunology ( IF 5.4 ) Pub Date : 2020-02-18 , DOI: 10.1002/eji.201948414 Hongxiang Lu 1, 2 , Rong Chen 1, 2 , Prince Amoah Barnie 1 , Yu Tian 1, 2 , Shiqing Zhang 1, 2 , Huaxi Xu 2 , Subrata Chakrabarti 3 , Zhaoliang Su 1, 2, 4
Affiliation
|
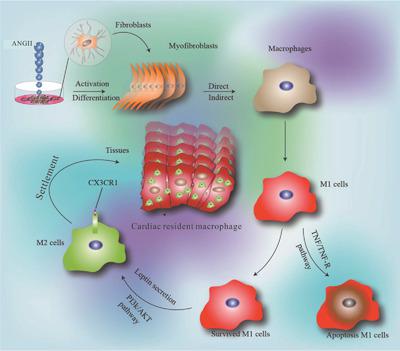
Resident cardiac macrophages play important roles in homeostasis, maintenance of cardiac function, and tissue repair. After cardiac injury, monocytes infiltrate the tissue, undergo phenotypic and functional changes, and are involved in inflammatory injury and functional remodelling. However, the fate of cardiac infiltrating/polarized macrophages and the relationship between these cells and resident cardiac macrophage replenishment following injury remain unclear. Our results showed that angiotensin II induces cardiac fibroblast transdifferentiation into cardiac myofibroblasts (MFBs). In cocultures with MFBs and murine macrophages, the MFBs promoted macrophage polarization to M1 phenotype, followed by selective apoptosis, which was associated with TNF/TNFR1 axis and independent of NO production. Surprisingly, after 36 h of coculture, the surviving macrophages were converted to M2 phenotype and settled in heart, which was dependent on leptin produced by MFBs or polarized macrophages via the PI3K or Akt pathway. CCR2+CD45.2+ cells adoptively transferred into CD45.1+ mice with viral myocarditis, differentiated into CD45.2+CCR2+CX3CR1+ M2 cells during the resolution of inflammation and settled within the heart. Our data highlight a novel mechanism related to the renewal or replenishment of cardiac resident macrophages following cardiac injury; and suggest that transdifferentiation of cardiac fibroblasts may promote the resolution of inflammation.
中文翻译:

成纤维细胞转分化促进小鼠心脏损伤后M1巨噬细胞的转化和心脏常驻巨噬细胞的补充。
常驻心脏巨噬细胞在体内稳态,心脏功能维持和组织修复中起重要作用。心脏损伤后,单核细胞浸润组织,发生表型和功能变化,并参与炎症性损伤和功能重塑。然而,心脏浸润/极化巨噬细胞的命运以及这些细胞与损伤后常驻心脏巨噬细胞补充之间的关系仍不清楚。我们的结果表明,血管紧张素II诱导心脏成纤维细胞向心肌成纤维细胞(MFBs)的转分化。在与MFB和鼠巨噬细胞共培养中,MFB促进巨噬细胞极化至M1表型,然后是选择性凋亡,这与TNF / TNFR1轴相关且与NO的产生无关。令人惊讶的是,共培养36小时后,存活的巨噬细胞转化为M2表型并在心脏定居,这依赖于MFB或极化巨噬细胞通过PI3K或Akt途径产生的瘦素。CCR2+ CD45.2 +细胞过继转移到患有病毒性心肌炎的CD45.1 +小鼠中,在炎症消退期间分化为CD45.2 + CCR2 + CX3CR1 + M2细胞,并在心脏内沉降。我们的数据强调了与心脏损伤后心脏常驻巨噬细胞的更新或补充有关的新机制。并提示心脏成纤维细胞的转分化可能促进炎症的消退。
更新日期:2020-02-18
中文翻译:

成纤维细胞转分化促进小鼠心脏损伤后M1巨噬细胞的转化和心脏常驻巨噬细胞的补充。
常驻心脏巨噬细胞在体内稳态,心脏功能维持和组织修复中起重要作用。心脏损伤后,单核细胞浸润组织,发生表型和功能变化,并参与炎症性损伤和功能重塑。然而,心脏浸润/极化巨噬细胞的命运以及这些细胞与损伤后常驻心脏巨噬细胞补充之间的关系仍不清楚。我们的结果表明,血管紧张素II诱导心脏成纤维细胞向心肌成纤维细胞(MFBs)的转分化。在与MFB和鼠巨噬细胞共培养中,MFB促进巨噬细胞极化至M1表型,然后是选择性凋亡,这与TNF / TNFR1轴相关且与NO的产生无关。令人惊讶的是,共培养36小时后,存活的巨噬细胞转化为M2表型并在心脏定居,这依赖于MFB或极化巨噬细胞通过PI3K或Akt途径产生的瘦素。CCR2+ CD45.2 +细胞过继转移到患有病毒性心肌炎的CD45.1 +小鼠中,在炎症消退期间分化为CD45.2 + CCR2 + CX3CR1 + M2细胞,并在心脏内沉降。我们的数据强调了与心脏损伤后心脏常驻巨噬细胞的更新或补充有关的新机制。并提示心脏成纤维细胞的转分化可能促进炎症的消退。


























 京公网安备 11010802027423号
京公网安备 11010802027423号