Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
DNA methylation: A mechanism for sustained alteration of KIR4.1 expression following central nervous system insult.
Glia ( IF 6.2 ) Pub Date : 2020-02-18 , DOI: 10.1002/glia.23797 Jessica L Boni 1, 2 , Uri Kahanovitch 2 , Sinifunanya E Nwaobi 1, 3 , Candace L Floyd 4, 5 , Michelle L Olsen 1, 2
Glia ( IF 6.2 ) Pub Date : 2020-02-18 , DOI: 10.1002/glia.23797 Jessica L Boni 1, 2 , Uri Kahanovitch 2 , Sinifunanya E Nwaobi 1, 3 , Candace L Floyd 4, 5 , Michelle L Olsen 1, 2
Affiliation
|
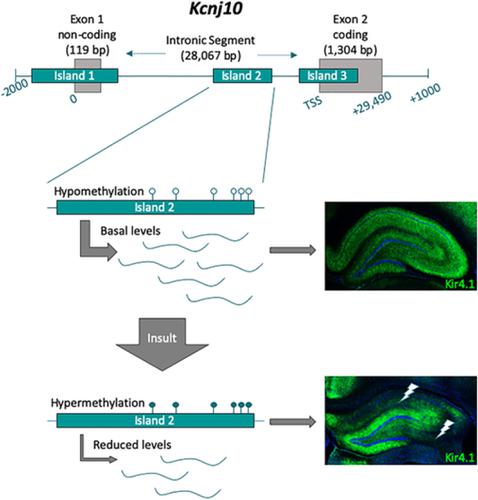
Kir4.1, a glial-specific inwardly rectifying potassium channel, is implicated in astrocytic maintenance of K+ homeostasis. Underscoring the role of Kir4.1 in central nervous system (CNS) functioning, genetic mutations in KCNJ10, the gene which encodes Kir4.1, causes seizures, ataxia and developmental disability in humans. Kir4.1 protein and mRNA loss are consistently observed in CNS injury and neurological diseases linked to hyperexcitability and neuronal dysfunction, leading to the notion that Kir4.1 represents an attractive therapeutic target. Despite this, little is understood regarding the mechanisms that underpin this downregulation. Previous work by our lab revealed that DNA hypomethylation of the Kcnj10 gene functions to regulate mRNA levels during astrocyte maturation whereas hypermethylation in vitro led to decreased promoter activity. In the present study, we utilized two vastly different injury models with known acute and chronic loss of Kir4.1 protein and mRNA to evaluate the methylation status of Kcnj10 as a candidate molecular mechanism for reduced transcription and subsequent protein loss. Examining whole hippocampal tissue and isolated astrocytes, in a lithium-pilocarpine model of epilepsy, we consistently identified hypermethylation of CpG island two, which resides in the large intronic region spanning the Kcnj10 gene. Strikingly similar results were observed using the second injury paradigm, a fifth cervical (C5) vertebral hemi-contusion model of spinal cord injury. Our previous work indicates the same gene region is significantly hypomethylated when transcription increases during astrocyte maturation. Our results suggest that DNA methylation can bidirectionally modulate Kcnj10 transcription and may represent a targetable molecular mechanism for the restoring astroglial Kir4.1 expression following CNS insult.
中文翻译:

DNA 甲基化:中枢神经系统损伤后 KIR4.1 表达持续改变的机制。
Kir4.1 是一种神经胶质细胞特异性的内向整流钾通道,与星形胶质细胞 K+ 稳态的维持有关。编码 Kir4.1 的基因 KCNJ10 的基因突变会导致人类癫痫发作、共济失调和发育障碍,这凸显了 Kir4.1 在中枢神经系统 (CNS) 功能中的作用。Kir4.1 蛋白和 mRNA 丢失在与过度兴奋和神经元功能障碍相关的中枢神经系统损伤和神经系统疾病中持续观察到,因此人们认为 Kir4.1 是一个有吸引力的治疗靶点。尽管如此,人们对于支持这种下调的机制知之甚少。我们实验室之前的工作表明,Kcnj10 基因的 DNA 低甲基化可在星形胶质细胞成熟过程中调节 mRNA 水平,而体外高甲基化会导致启动子活性降低。在本研究中,我们利用两种截然不同的损伤模型(已知 Kir4.1 蛋白和 mRNA 的急性和慢性丢失)来评估 Kcnj10 的甲基化状态,作为减少转录和随后的蛋白质丢失的候选分子机制。在锂-毛果芸香碱癫痫模型中检查整个海马组织和分离的星形胶质细胞,我们一致发现了 CpG 岛 2 的高甲基化,该岛位于跨越 Kcnj10 基因的大内含子区域。使用第二种损伤范例,即脊髓损伤的第五颈椎 (C5) 椎体半挫伤模型,观察到了惊人相似的结果。我们之前的工作表明,当星形胶质细胞成熟期间转录增加时,同一基因区域显着低甲基化。我们的结果表明,DNA 甲基化可以双向调节 Kcnj10 转录,并可能代表中枢神经系统损伤后恢复星形胶质细胞 Kir4.1 表达的可靶向分子机制。
更新日期:2020-02-18
中文翻译:

DNA 甲基化:中枢神经系统损伤后 KIR4.1 表达持续改变的机制。
Kir4.1 是一种神经胶质细胞特异性的内向整流钾通道,与星形胶质细胞 K+ 稳态的维持有关。编码 Kir4.1 的基因 KCNJ10 的基因突变会导致人类癫痫发作、共济失调和发育障碍,这凸显了 Kir4.1 在中枢神经系统 (CNS) 功能中的作用。Kir4.1 蛋白和 mRNA 丢失在与过度兴奋和神经元功能障碍相关的中枢神经系统损伤和神经系统疾病中持续观察到,因此人们认为 Kir4.1 是一个有吸引力的治疗靶点。尽管如此,人们对于支持这种下调的机制知之甚少。我们实验室之前的工作表明,Kcnj10 基因的 DNA 低甲基化可在星形胶质细胞成熟过程中调节 mRNA 水平,而体外高甲基化会导致启动子活性降低。在本研究中,我们利用两种截然不同的损伤模型(已知 Kir4.1 蛋白和 mRNA 的急性和慢性丢失)来评估 Kcnj10 的甲基化状态,作为减少转录和随后的蛋白质丢失的候选分子机制。在锂-毛果芸香碱癫痫模型中检查整个海马组织和分离的星形胶质细胞,我们一致发现了 CpG 岛 2 的高甲基化,该岛位于跨越 Kcnj10 基因的大内含子区域。使用第二种损伤范例,即脊髓损伤的第五颈椎 (C5) 椎体半挫伤模型,观察到了惊人相似的结果。我们之前的工作表明,当星形胶质细胞成熟期间转录增加时,同一基因区域显着低甲基化。我们的结果表明,DNA 甲基化可以双向调节 Kcnj10 转录,并可能代表中枢神经系统损伤后恢复星形胶质细胞 Kir4.1 表达的可靶向分子机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号