当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Turning main-group element magnesium into a highly active electrocatalyst for oxygen reduction reaction.
Nature Communications ( IF 16.6 ) Pub Date : 2020-02-18 , DOI: 10.1038/s41467-020-14565-w Shuai Liu 1 , Zedong Li 1 , Changlai Wang 1 , Weiwei Tao 2 , Minxue Huang 1 , Ming Zuo 1 , Yang Yang 1 , Kang Yang 1 , Lijuan Zhang 3 , Shi Chen 1 , Pengping Xu 1 , Qianwang Chen 1, 4
Nature Communications ( IF 16.6 ) Pub Date : 2020-02-18 , DOI: 10.1038/s41467-020-14565-w Shuai Liu 1 , Zedong Li 1 , Changlai Wang 1 , Weiwei Tao 2 , Minxue Huang 1 , Ming Zuo 1 , Yang Yang 1 , Kang Yang 1 , Lijuan Zhang 3 , Shi Chen 1 , Pengping Xu 1 , Qianwang Chen 1, 4
Affiliation
|
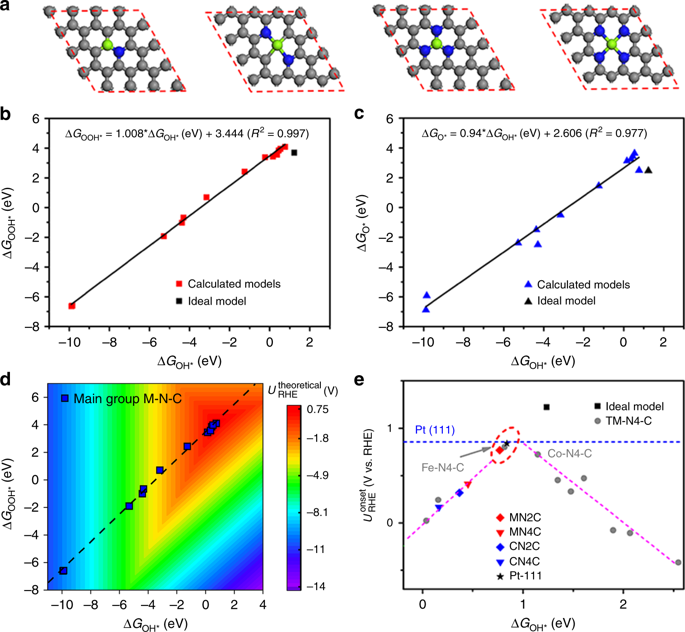
It is known that the main-group metals and their related materials show poor catalytic activity due to a broadened single resonance derived from the interaction of valence orbitals of adsorbates with the broad sp-band of main-group metals. However, Mg cofactors existing in enzymes are extremely active in biochemical reactions. Our density function theory calculations reveal that the catalytic activity of the main-group metals (Mg, Al and Ca) in oxygen reduction reaction is severely hampered by the tight-bonding of active centers with hydroxyl group intermediate, while the Mg atom coordinated to two nitrogen atoms has the near-optimal adsorption strength with intermediate oxygen species by the rise of p-band center position compared to other coordination environments. We experimentally demonstrate that the atomically dispersed Mg cofactors incorporated within graphene framework exhibits a strikingly high half-wave potential of 910 mV in alkaline media, turning a s/p-band metal into a highly active electrocatalyst.
中文翻译:

将主族元素镁转变为用于氧还原反应的高活性电催化剂。
众所周知,主族金属及其相关材料的催化活性差,这是由于从被吸附物的价态轨道与主族金属的宽sp带相互作用产生的单个共振变宽了。但是,存在于酶中的Mg辅助因子在生化反应中极为活跃。我们的密度泛函理论计算表明,活性中心与羟基中间体的紧密键合严重阻碍了主族金属(Mg,Al和Ca)在氧还原反应中的催化活性,而Mg原子配位为两个与其他配位环境相比,通过p带中心位置的升高,氮原子对中等氧物种的吸附强度接近最佳。
更新日期:2020-02-18
中文翻译:

将主族元素镁转变为用于氧还原反应的高活性电催化剂。
众所周知,主族金属及其相关材料的催化活性差,这是由于从被吸附物的价态轨道与主族金属的宽sp带相互作用产生的单个共振变宽了。但是,存在于酶中的Mg辅助因子在生化反应中极为活跃。我们的密度泛函理论计算表明,活性中心与羟基中间体的紧密键合严重阻碍了主族金属(Mg,Al和Ca)在氧还原反应中的催化活性,而Mg原子配位为两个与其他配位环境相比,通过p带中心位置的升高,氮原子对中等氧物种的吸附强度接近最佳。



























 京公网安备 11010802027423号
京公网安备 11010802027423号