当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
pH Switchable Nanoplatform for In Vivo Persistent Luminescence Imaging and Precise Photothermal Therapy of Bacterial Infection
Advanced Functional Materials ( IF 19.0 ) Pub Date : 2020-02-16 , DOI: 10.1002/adfm.201909042 Li‐Xia Yan 1, 2 , Li‐Jian Chen 1, 2 , Xu Zhao 1, 2 , Xiu‐Ping Yan 1, 2, 3
Advanced Functional Materials ( IF 19.0 ) Pub Date : 2020-02-16 , DOI: 10.1002/adfm.201909042 Li‐Xia Yan 1, 2 , Li‐Jian Chen 1, 2 , Xu Zhao 1, 2 , Xiu‐Ping Yan 1, 2, 3
Affiliation
|
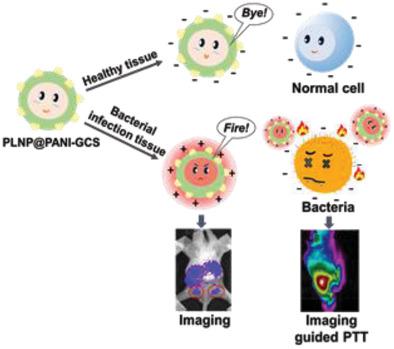
Photothermal therapy (PTT) is one of the most promising approaches to combat multidrug‐resistant bacteria with less potential to induce resistance and systemic toxicity. However, uncontrollable distribution of photothermal agents leads to lethal temperatures for normal cells, and failure to offer timely and effective antibacterial stewardship. A pH switchable nanoplatform for persistent luminescence imaging‐guided precise PTT to selectively destroy only pathological cells while protecting nearby normal cells in bacterial infected microenvironment is shown. The PLNP@PANI‐GCS is fabricated by grafting polyaniline (PANI) and glycol chitosan (GCS) onto the surface of persistent luminescence nanoparticles (PLNPs). It takes advantage of the long persistent luminescence of PLNPs to realize autofluorescence‐free imaging, the pH‐dependent light–heat conversion property of PANI to get a stronger photothermal effect at pH 6.5 than pH 7.4, and the pH environment responsive surface charge transition of GCS. Consequently, PLNP@PANI‐GCS enables effective response to bacterial‐infected acid region and electrostatic bonding to bacteria in vivo, ensuring the spatial accuracy of near‐infrared light irradiation and specific heating directly to bacteria. In vivo imaging‐guided PTT to bacterial infection abscess shows effective treatment. PLNP@PANI‐GCS has great potential in treating multidrug‐resistant bacterial infection with low possibility of developing microbial drug resistance and little harm to normal cells.
中文翻译:

pH可调纳米平台用于体内持久发光成像和细菌感染的精确光热疗法。
光热疗法(PTT)是与多药耐药细菌作斗争的最有前途的方法之一,其诱导耐药性和全身毒性的潜力较小。但是,光热剂的不可控分布会导致正常细胞致死的温度,并且无法提供及时有效的抗菌素管理。显示了用于持续发光成像引导的精确pH转换的pH可切换纳米平台,可以选择性地仅破坏病理细胞,同时在细菌感染的微环境中保护附近的正常细胞。PLNP @ PANI-GCS是通过将聚苯胺(PANI)和乙二醇壳聚糖(GCS)接枝到持久发光纳米粒子(PLNP)的表面上而制成的。它利用PLNP的持久发光来实现无自发荧光成像,PANI的依赖于pH的光热转换特性在pH 6.5时比pH 7.4时获得更强的光热效应,并且pH环境响应GCS的表面电荷转变。因此,PLNP @ PANI‐GCS能够对细菌感染的酸性区域做出有效反应,并在体内与细菌静电结合,从而确保近红外光辐照和直接加热细菌的空间准确性。体内影像引导的PTT对细菌感染脓肿显示出有效的治疗方法。PLNP @ PANI‐GCS在治疗多药耐药细菌感染方面具有巨大潜力,对微生物产生耐药性的可能性很小,并且对正常细胞的危害很小。PLNP @ PANI‐GCS可对细菌感染的酸性区域做出有效反应,并在体内与细菌静电结合,从而确保近红外光照射和直接对细菌的特异性加热的空间精度。体内影像引导的PTT对细菌感染脓肿显示出有效的治疗方法。PLNP @ PANI‐GCS在治疗多药耐药细菌感染方面具有巨大潜力,对微生物产生耐药性的可能性很小,并且对正常细胞的危害很小。PLNP @ PANI‐GCS可对细菌感染的酸性区域做出有效反应,并在体内与细菌静电结合,从而确保近红外光照射和直接对细菌的特异性加热的空间精度。体内影像引导的PTT对细菌感染脓肿显示出有效的治疗方法。PLNP @ PANI‐GCS在治疗多药耐药细菌感染方面具有巨大潜力,对微生物产生耐药性的可能性很小,并且对正常细胞的危害很小。
更新日期:2020-04-06
中文翻译:

pH可调纳米平台用于体内持久发光成像和细菌感染的精确光热疗法。
光热疗法(PTT)是与多药耐药细菌作斗争的最有前途的方法之一,其诱导耐药性和全身毒性的潜力较小。但是,光热剂的不可控分布会导致正常细胞致死的温度,并且无法提供及时有效的抗菌素管理。显示了用于持续发光成像引导的精确pH转换的pH可切换纳米平台,可以选择性地仅破坏病理细胞,同时在细菌感染的微环境中保护附近的正常细胞。PLNP @ PANI-GCS是通过将聚苯胺(PANI)和乙二醇壳聚糖(GCS)接枝到持久发光纳米粒子(PLNP)的表面上而制成的。它利用PLNP的持久发光来实现无自发荧光成像,PANI的依赖于pH的光热转换特性在pH 6.5时比pH 7.4时获得更强的光热效应,并且pH环境响应GCS的表面电荷转变。因此,PLNP @ PANI‐GCS能够对细菌感染的酸性区域做出有效反应,并在体内与细菌静电结合,从而确保近红外光辐照和直接加热细菌的空间准确性。体内影像引导的PTT对细菌感染脓肿显示出有效的治疗方法。PLNP @ PANI‐GCS在治疗多药耐药细菌感染方面具有巨大潜力,对微生物产生耐药性的可能性很小,并且对正常细胞的危害很小。PLNP @ PANI‐GCS可对细菌感染的酸性区域做出有效反应,并在体内与细菌静电结合,从而确保近红外光照射和直接对细菌的特异性加热的空间精度。体内影像引导的PTT对细菌感染脓肿显示出有效的治疗方法。PLNP @ PANI‐GCS在治疗多药耐药细菌感染方面具有巨大潜力,对微生物产生耐药性的可能性很小,并且对正常细胞的危害很小。PLNP @ PANI‐GCS可对细菌感染的酸性区域做出有效反应,并在体内与细菌静电结合,从而确保近红外光照射和直接对细菌的特异性加热的空间精度。体内影像引导的PTT对细菌感染脓肿显示出有效的治疗方法。PLNP @ PANI‐GCS在治疗多药耐药细菌感染方面具有巨大潜力,对微生物产生耐药性的可能性很小,并且对正常细胞的危害很小。


























 京公网安备 11010802027423号
京公网安备 11010802027423号