当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
2‐Benzoyl Thienothiazoles from Annulation of C−H Bonds in Acetophenone Oximes
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-02-25 , DOI: 10.1002/ajoc.202000046 Phuc H. Pham 1, 2 , Khang X. Nguyen 1, 2 , Ninh P. Nguyen 1, 2 , Hoai T. B. Pham 1, 2, 3 , Tung T. Nguyen 1, 2 , Nam T. S. Phan 1, 2
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-02-25 , DOI: 10.1002/ajoc.202000046 Phuc H. Pham 1, 2 , Khang X. Nguyen 1, 2 , Ninh P. Nguyen 1, 2 , Hoai T. B. Pham 1, 2, 3 , Tung T. Nguyen 1, 2 , Nam T. S. Phan 1, 2
Affiliation
|
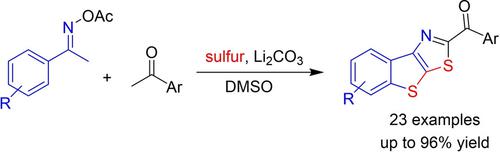
We report a method for coupling of acetophenone oxime acetates, C−H bonds α to ketones, and elemental sulfur. The reactions proceeded in the presence of Li2CO3 (base) and DMSO (solvent) to afford 2‐benzoyl benzothienothiazoles. Functionalities including halogen, protected alcohol, and heterocycle groups were compatible with reaction conditions. Use of methylene C−H bonds in β‐keto esters is also viable. This marks a rare example for mild synthesis of S‐heterocycles from ketoxime acetates.
中文翻译:

苯乙酮肟中C-H键环化的2-苯甲酰基噻吩并噻唑
我们报告了一种苯乙酮肟乙酸酯,CH键与酮和元素硫的CH键偶联方法。反应在Li 2 CO 3(碱)和DMSO(溶剂)存在下进行,得到2-苯甲酰基苯并噻吩并噻唑。包括卤素,受保护的醇和杂环基在内的功能与反应条件兼容。在β-酮酸酯中使用亚甲基CH键也是可行的。这标志着由醋酸酮肟轻度合成S杂环的罕见例子。
更新日期:2020-04-21
中文翻译:

苯乙酮肟中C-H键环化的2-苯甲酰基噻吩并噻唑
我们报告了一种苯乙酮肟乙酸酯,CH键与酮和元素硫的CH键偶联方法。反应在Li 2 CO 3(碱)和DMSO(溶剂)存在下进行,得到2-苯甲酰基苯并噻吩并噻唑。包括卤素,受保护的醇和杂环基在内的功能与反应条件兼容。在β-酮酸酯中使用亚甲基CH键也是可行的。这标志着由醋酸酮肟轻度合成S杂环的罕见例子。


























 京公网安备 11010802027423号
京公网安备 11010802027423号