Nature Immunology ( IF 30.5 ) Pub Date : 2020-02-17 , DOI: 10.1038/s41590-019-0589-5 Haiping Wang 1, 2 , Fabien Franco 1, 2 , Yao-Chen Tsui 1, 2 , Xin Xie 3 , Marcel P Trefny 4 , Roberta Zappasodi 5, 6 , Syed Raza Mohmood 3 , Juan Fernández-García 7, 8 , Chin-Hsien Tsai 1, 2 , Isabell Schulze 5, 6 , Florence Picard 1, 2 , Etienne Meylan 9 , Roy Silverstein 10 , Ira Goldberg 11 , Sarah-Maria Fendt 7, 8 , Jedd D Wolchok 5, 6, 12, 13 , Taha Merghoub 5, 6, 12 , Camilla Jandus 1, 2 , Alfred Zippelius 4, 14 , Ping-Chih Ho 1, 2
|
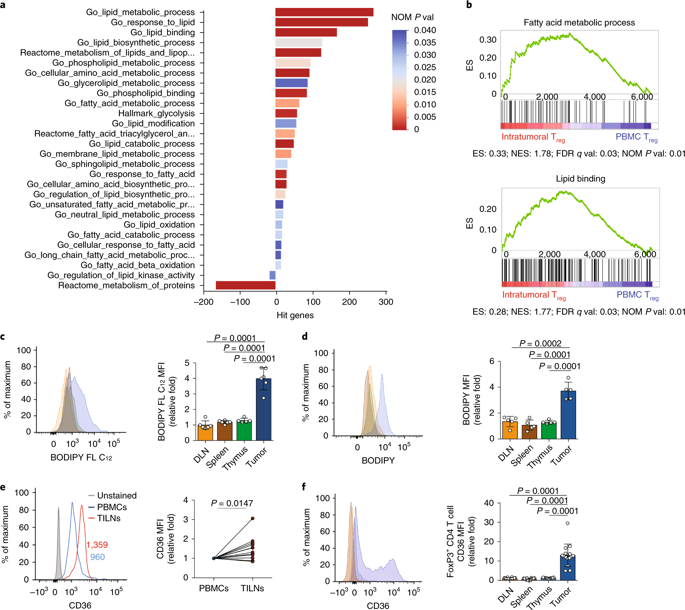
Depleting regulatory T cells (Treg cells) to counteract immunosuppressive features of the tumor microenvironment (TME) is an attractive strategy for cancer treatment; however, autoimmunity due to systemic impairment of their suppressive function limits its therapeutic potential. Elucidating approaches that specifically disrupt intratumoral Treg cells is direly needed for cancer immunotherapy. We found that CD36 was selectively upregulated in intrautumoral Treg cells as a central metabolic modulator. CD36 fine-tuned mitochondrial fitness via peroxisome proliferator-activated receptor-β signaling, programming Treg cells to adapt to a lactic acid-enriched TME. Genetic ablation of Cd36 in Treg cells suppressed tumor growth accompanied by a decrease in intratumoral Treg cells and enhancement of antitumor activity in tumor-infiltrating lymphocytes without disrupting immune homeostasis. Furthermore, CD36 targeting elicited additive antitumor responses with anti-programmed cell death protein 1 therapy. Our findings uncover the unexplored metabolic adaptation that orchestrates the survival and functions of intratumoral Treg cells, and the therapeutic potential of targeting this pathway for reprogramming the TME.
中文翻译:

CD36 介导的代谢适应支持调节性 T 细胞在肿瘤中的存活和功能
消耗调节性 T 细胞 (T reg细胞) 以抵消肿瘤微环境 (TME) 的免疫抑制特征是一种有吸引力的癌症治疗策略;然而,由于其抑制功能的系统性损害导致的自身免疫限制了其治疗潜力。癌症免疫治疗迫切需要阐明特异性破坏肿瘤内 T reg细胞的方法。我们发现 CD36 在肿瘤内 T reg细胞中作为中枢代谢调节剂选择性上调。CD36 通过过氧化物酶体增殖物激活受体-β 信号传导微调线粒体适应性,编程 T reg细胞以适应富含乳酸的 TME。T reg中Cd36的基因消融细胞抑制肿瘤生长,伴随着肿瘤内 T reg细胞的减少和肿瘤浸润淋巴细胞的抗肿瘤活性增强,而不会破坏免疫稳态。此外,针对 CD36 的抗程序性细胞死亡蛋白 1 疗法引发了附加的抗肿瘤反应。我们的研究结果揭示了协调肿瘤内 T reg细胞的存活和功能的未探索的代谢适应,以及靶向该途径重编程 TME 的治疗潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号