Nature Chemical Biology ( IF 14.8 ) Pub Date : 2020-02-17 , DOI: 10.1038/s41589-019-0462-8 Alexandr A Kapralov 1 , Qin Yang 2 , Haider H Dar 1 , Yulia Y Tyurina 1 , Tamil S Anthonymuthu 2 , Rina Kim 3, 4 , Claudette M St Croix 5 , Karolina Mikulska-Ruminska 6, 7 , Bing Liu 6 , Indira H Shrivastava 1, 6 , Vladimir A Tyurin 1 , Hsiu-Chi Ting 1 , Yijen L Wu 8 , Yuan Gao 2 , Galina V Shurin 1 , Margarita A Artyukhova 1, 9 , Liubov A Ponomareva 1, 9 , Peter S Timashev 9 , Rosario M Domingues 10, 11 , Detcho A Stoyanovsky 1 , Joel S Greenberger 12 , Rama K Mallampalli 13 , Ivet Bahar 6 , Dmitry I Gabrilovich 3 , Hülya Bayır 1, 2 , Valerian E Kagan 1, 9, 12, 14, 15
|
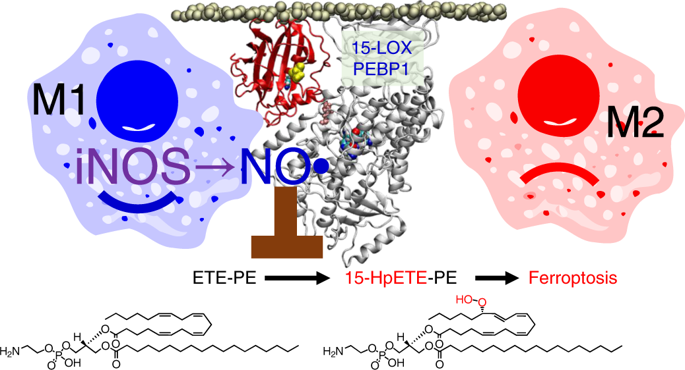
Ferroptotic death is the penalty for losing control over three processes—iron metabolism, lipid peroxidation and thiol regulation—that are common in the pro-inflammatory environment where professional phagocytes fulfill their functions and yet survive. We hypothesized that redox reprogramming of 15-lipoxygenase (15-LOX) during the generation of pro-ferroptotic signal 15-hydroperoxy-eicosa-tetra-enoyl-phosphatidylethanolamine (15-HpETE-PE) modulates ferroptotic endurance. Here, we have discovered that inducible nitric oxide synthase (iNOS)/NO•-enrichment of activated M1 (but not alternatively activated M2) macrophages/microglia modulates susceptibility to ferroptosis. Genetic or pharmacologic depletion/inactivation of iNOS confers sensitivity on M1 cells, whereas NO• donors empower resistance of M2 cells to ferroptosis. In vivo, M1 phagocytes, in comparison to M2 phagocytes, exert higher resistance to pharmacologically induced ferroptosis. This resistance is diminished in iNOS-deficient cells in the pro-inflammatory conditions of brain trauma or the tumour microenvironment. The nitroxygenation of eicosatetraenoyl (ETE)-PE intermediates and oxidatively truncated species by NO• donors and/or suppression of NO• production by iNOS inhibitors represent a novel redox mechanism of regulation of ferroptosis in pro-inflammatory conditions.
中文翻译:

氧化还原脂质重编程命令巨噬细胞和小胶质细胞对铁死亡的易感性
Ferroptotic 死亡是失去对三个过程(铁代谢、脂质过氧化和硫醇调节)的控制的惩罚,这三个过程在促炎环境中很常见,在这个环境中,专业的吞噬细胞履行其功能并存活下来。我们假设在促铁死亡信号 15-hydroperoxy-eicosa-tetra-enyl-phosphatidylethanolamine (15-HpETE-PE) 的产生过程中 15-脂氧合酶 (15-LOX) 的氧化还原重编程调节铁死亡耐力。在这里,我们发现诱导型一氧化氮合酶 (iNOS)/NO •富集活化的 M1(但不是活化的 M2)巨噬细胞/小胶质细胞可调节对铁死亡的易感性。iNOS 的遗传或药理学消耗/失活赋予 M1 细胞敏感性,而 NO •捐赠者增强 M2 细胞对铁死亡的抵抗力。在体内,与 M2 吞噬细胞相比,M1 吞噬细胞对药理学诱导的铁死亡具有更高的抵抗力。在脑外伤或肿瘤微环境的促炎条件下,iNOS 缺陷细胞的这种抵抗力会减弱。NO •供体对二十碳四烯酰 (ETE)-PE 中间体和氧化截短物质的氮氧化作用和/或 iNOS 抑制剂对 NO •产生的抑制代表了促炎条件下调节铁死亡的一种新型氧化还原机制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号