当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploring the Histone Acylome through Incorporation of γ-Thialysine on Histone Tails.
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2020-02-14 , DOI: 10.1021/acs.bioconjchem.0c00012 Giordano Proietti 1 , Giorgio Rainone 1 , Jordi C J Hintzen 1 , Jasmin Mecinović 1
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2020-02-14 , DOI: 10.1021/acs.bioconjchem.0c00012 Giordano Proietti 1 , Giorgio Rainone 1 , Jordi C J Hintzen 1 , Jasmin Mecinović 1
Affiliation
|
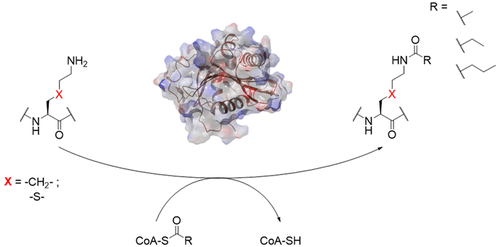
Histone lysine acetyltransferases (KATs) catalyze the transfer of the acetyl group from acetyl Coenzyme A to lysine residues in histones and nonhistone proteins. Here, we report biomolecular studies on epigenetic acetylation and related acylation reactions of lysine and γ-thialysine, a cysteine-derived lysine mimic, which can be site-specifically introduced to histone peptides and histone proteins. Enzyme assays demonstrate that human KATs catalyze an efficient acetylation and propionylation of histone peptides that possess lysine and γ-thialysine. Enzyme kinetics analyses reveal that lysine- and γ-thialysine-containing histone peptides exhibit indistinguishable Km values, whereas small differences in kcat values were observed. This work highlights that γ-thialysine may act as a representative and easily accessible lysine mimic for chemical and biochemical examinations of post-translationally modified histones.
中文翻译:

通过在组蛋白尾巴上掺入γ-硫赖氨酸来探索组蛋白酰基。
组蛋白赖氨酸乙酰基转移酶(KAT)催化乙酰基从乙酰辅酶A转移到组蛋白和非组蛋白中的赖氨酸残基上。在这里,我们报道了赖氨酸和γ-硫代赖氨酸(一种半胱氨酸衍生的赖氨酸模拟物)的表观遗传学乙酰化和相关酰化反应的生物分子研究,可以将其位点特异性引入组蛋白肽和组蛋白中。酶法测定表明,人KAT可催化具有赖氨酸和γ-硫代赖氨酸的组蛋白肽的有效乙酰化和丙酰化。酶动力学分析表明,含有赖氨酸和γ-硫代赖氨酸的组蛋白肽的Km值无法区分,而kcat值则存在微小差异。
更新日期:2020-02-28
中文翻译:

通过在组蛋白尾巴上掺入γ-硫赖氨酸来探索组蛋白酰基。
组蛋白赖氨酸乙酰基转移酶(KAT)催化乙酰基从乙酰辅酶A转移到组蛋白和非组蛋白中的赖氨酸残基上。在这里,我们报道了赖氨酸和γ-硫代赖氨酸(一种半胱氨酸衍生的赖氨酸模拟物)的表观遗传学乙酰化和相关酰化反应的生物分子研究,可以将其位点特异性引入组蛋白肽和组蛋白中。酶法测定表明,人KAT可催化具有赖氨酸和γ-硫代赖氨酸的组蛋白肽的有效乙酰化和丙酰化。酶动力学分析表明,含有赖氨酸和γ-硫代赖氨酸的组蛋白肽的Km值无法区分,而kcat值则存在微小差异。


























 京公网安备 11010802027423号
京公网安备 11010802027423号