当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comparing Nonbonded Metal Ion Models in the Divalent Cation Binding Protein PsaA.
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2020-02-26 , DOI: 10.1021/acs.jctc.9b01180 Hugo MacDermott-Opeskin 1 , Christopher A McDevitt 2 , Megan L O'Mara 1
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2020-02-26 , DOI: 10.1021/acs.jctc.9b01180 Hugo MacDermott-Opeskin 1 , Christopher A McDevitt 2 , Megan L O'Mara 1
Affiliation
|
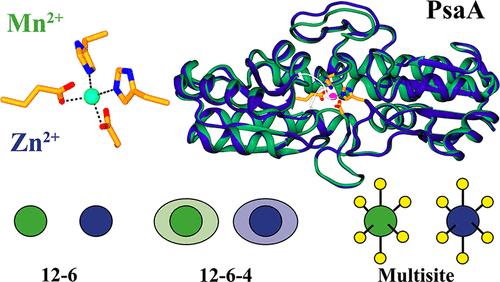
Divalent metal cations are essential for many biological processes; however, accurately modeling divalent metal ions has proved a significant challenge for molecular dynamics force fields. Here we show that the choice of ion model influences the observed dynamics in PsaA, a metal binding protein from Streptococcus pneumoniae. We conduct extensive unbiased simulations and free energy calculations of PsaA bound to its cognate ligand Mn2+ and inhibitory ligand Zn2+ using three nonbonded ion models: a 12-6 model, a 12-6-4 model, and a multisite model. The observed coordination geometries and metal binding dynamics are sensitive to the choice of ion model, with the most dramatic differences observed in free energy calculations of ion release. We show that the conformational ensemble of Mn-bound PsaA is more similar to the crystallographic metal bound open state. This work extends the current model of PsaA metal binding and provides a framework for the rationalization of experimentally determined metal binding behavior. Our findings support the use of the 12-6-4 ion model for further simulations of divalent cation binding proteins.
中文翻译:

比较二价阳离子结合蛋白PsaA中的非键金属离子模型。
二价金属阳离子对于许多生物过程至关重要。然而,对二价金属离子进行精确建模已证明对分子动力学力场提出了重大挑战。在这里,我们显示离子模型的选择会影响PsaA(一种来自肺炎链球菌的金属结合蛋白)中观察到的动力学。我们使用三个非键合离子模型:12-6模型,12-6-4模型和多位点模型,对与其同源配体Mn2 +和抑制性配体Zn2 +结合的PsaA进行了广泛的无偏模拟和自由能计算。观察到的配位几何形状和金属结合动力学对离子模型的选择敏感,在离子释放的自由能计算中观察到最大的差异。我们表明,Mn结合的PsaA的构象整体与晶体学的金属结合的开放态更相似。这项工作扩展了目前的PsaA金属结合模型,并为合理确定实验确定的金属结合行为提供了框架。我们的发现支持使用12-6-4离子模型进一步模拟二价阳离子结合蛋白。
更新日期:2020-02-27
中文翻译:

比较二价阳离子结合蛋白PsaA中的非键金属离子模型。
二价金属阳离子对于许多生物过程至关重要。然而,对二价金属离子进行精确建模已证明对分子动力学力场提出了重大挑战。在这里,我们显示离子模型的选择会影响PsaA(一种来自肺炎链球菌的金属结合蛋白)中观察到的动力学。我们使用三个非键合离子模型:12-6模型,12-6-4模型和多位点模型,对与其同源配体Mn2 +和抑制性配体Zn2 +结合的PsaA进行了广泛的无偏模拟和自由能计算。观察到的配位几何形状和金属结合动力学对离子模型的选择敏感,在离子释放的自由能计算中观察到最大的差异。我们表明,Mn结合的PsaA的构象整体与晶体学的金属结合的开放态更相似。这项工作扩展了目前的PsaA金属结合模型,并为合理确定实验确定的金属结合行为提供了框架。我们的发现支持使用12-6-4离子模型进一步模拟二价阳离子结合蛋白。


























 京公网安备 11010802027423号
京公网安备 11010802027423号