当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phosphoproteomics Reveals Novel Targets and Phosphoprotein Networks in Cell Cycle Mediated by Dsk1 Kinase.
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2020-02-16 , DOI: 10.1021/acs.jproteome.0c00027 Mei Wu 1 , Gang Feng 2 , Buyu Zhang 1 , Kaikun Xu 3 , Zhen Wang 1 , Sen Cheng 1 , Cheng Chang 3 , Aditi Vyas 4 , Zhaohua Tang 4 , Xiaoyun Liu 5
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2020-02-16 , DOI: 10.1021/acs.jproteome.0c00027 Mei Wu 1 , Gang Feng 2 , Buyu Zhang 1 , Kaikun Xu 3 , Zhen Wang 1 , Sen Cheng 1 , Cheng Chang 3 , Aditi Vyas 4 , Zhaohua Tang 4 , Xiaoyun Liu 5
Affiliation
|
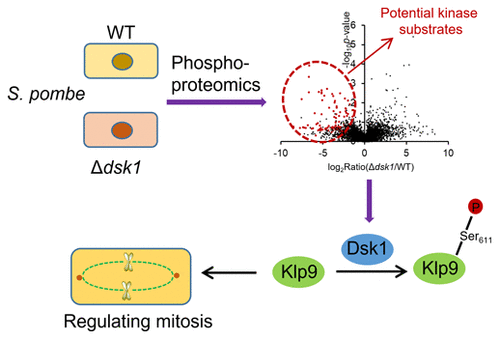
As the ortholog of human SR protein kinase 1 in fission yeast Schizosaccharomyces pombe, Dsk1 specifically phosphorylates SR proteins (serine/arginine-rich proteins) and promotes splicing of nonconsensus introns. The SRPK (SR protein-specific kinase) family performs highly conserved functions in eukaryotic cells including cell proliferation, differentiation, development, and apoptosis. Although Dsk1 was originally identified as a mitotic regulator, its specific targets involved in cell cycle have yet been unexplored. In this study, using a phosphoproteomics approach, we examined differential protein phosphorylation between wild-type cells and dsk1-deletion mutants. We found reduced phosphorylation of 149 peptides corresponding to 133 proteins in the dsk1-null cells. These proteins are involved in various cellular processes, including cytoskeleton organization and signal transduction, and specifically enriched in multiple steps of cell cycle control. Further, targeted MS analyses and in vitro biochemical assays established Cdr2 protein kinase and kinesin motor Klp9 as novel substrates of Dsk1, which function in cell size control for mitotic entry and in chromosome segregation for mitotic exit, respectively. The phosphoprotein networks mediated by Dsk1 reveal, for the first time, the molecular links connecting Dsk1 to mitotic phase transition, sister-chromatid segregation, and cytokinesis, providing further evidence of Dsk1's diverse influence on cell cycle progression and regulation.
中文翻译:

磷酸蛋白质组学揭示了 Dsk1 激酶介导的细胞周期中的新靶点和磷酸蛋白网络。
作为裂殖酵母粟酒裂殖酵母中人类 SR 蛋白激酶 1 的直系同源物,Dsk1 特异性磷酸化 SR 蛋白(富含丝氨酸/精氨酸的蛋白)并促进非一致内含子的剪接。SRPK(SR 蛋白特异性激酶)家族在真核细胞中执行高度保守的功能,包括细胞增殖、分化、发育和凋亡。虽然 Dsk1 最初被确定为有丝分裂调节剂,但其参与细胞周期的具体目标尚未探索。在这项研究中,我们使用磷酸蛋白质组学方法检查了野生型细胞和 dsk1 缺失突变体之间的差异蛋白磷酸化。我们发现 dsk1-null 细胞中对应于 133 种蛋白质的 149 种肽的磷酸化程度降低。这些蛋白质参与各种细胞过程,包括细胞骨架组织和信号转导,并特别丰富了细胞周期控制的多个步骤。此外,靶向 MS 分析和体外生化分析确定 Cdr2 蛋白激酶和驱动蛋白运动 Klp9 作为 Dsk1 的新底物,它们分别在有丝分裂进入的细胞大小控制和有丝分裂退出的染色体分离中起作用。由 Dsk1 介导的磷蛋白网络首次揭示了将 Dsk1 与有丝分裂相变、姐妹染色单体分离和胞质分裂联系起来的分子联系,进一步证明了 Dsk1 对细胞周期进程和调节的多种影响。靶向 MS 分析和体外生化分析确定 Cdr2 蛋白激酶和驱动蛋白运动 Klp9 作为 Dsk1 的新底物,它们分别在有丝分裂进入的细胞大小控制和有丝分裂退出的染色体分离中起作用。由 Dsk1 介导的磷蛋白网络首次揭示了连接 Dsk1 与有丝分裂相变、姐妹染色单体分离和胞质分裂的分子联系,进一步证明了 Dsk1 对细胞周期进程和调节的多种影响。靶向 MS 分析和体外生化分析确定 Cdr2 蛋白激酶和驱动蛋白运动 Klp9 作为 Dsk1 的新底物,它们分别在有丝分裂进入的细胞大小控制和有丝分裂退出的染色体分离中起作用。由 Dsk1 介导的磷蛋白网络首次揭示了连接 Dsk1 与有丝分裂相变、姐妹染色单体分离和胞质分裂的分子联系,进一步证明了 Dsk1 对细胞周期进程和调节的多种影响。
更新日期:2020-02-16
中文翻译:

磷酸蛋白质组学揭示了 Dsk1 激酶介导的细胞周期中的新靶点和磷酸蛋白网络。
作为裂殖酵母粟酒裂殖酵母中人类 SR 蛋白激酶 1 的直系同源物,Dsk1 特异性磷酸化 SR 蛋白(富含丝氨酸/精氨酸的蛋白)并促进非一致内含子的剪接。SRPK(SR 蛋白特异性激酶)家族在真核细胞中执行高度保守的功能,包括细胞增殖、分化、发育和凋亡。虽然 Dsk1 最初被确定为有丝分裂调节剂,但其参与细胞周期的具体目标尚未探索。在这项研究中,我们使用磷酸蛋白质组学方法检查了野生型细胞和 dsk1 缺失突变体之间的差异蛋白磷酸化。我们发现 dsk1-null 细胞中对应于 133 种蛋白质的 149 种肽的磷酸化程度降低。这些蛋白质参与各种细胞过程,包括细胞骨架组织和信号转导,并特别丰富了细胞周期控制的多个步骤。此外,靶向 MS 分析和体外生化分析确定 Cdr2 蛋白激酶和驱动蛋白运动 Klp9 作为 Dsk1 的新底物,它们分别在有丝分裂进入的细胞大小控制和有丝分裂退出的染色体分离中起作用。由 Dsk1 介导的磷蛋白网络首次揭示了将 Dsk1 与有丝分裂相变、姐妹染色单体分离和胞质分裂联系起来的分子联系,进一步证明了 Dsk1 对细胞周期进程和调节的多种影响。靶向 MS 分析和体外生化分析确定 Cdr2 蛋白激酶和驱动蛋白运动 Klp9 作为 Dsk1 的新底物,它们分别在有丝分裂进入的细胞大小控制和有丝分裂退出的染色体分离中起作用。由 Dsk1 介导的磷蛋白网络首次揭示了连接 Dsk1 与有丝分裂相变、姐妹染色单体分离和胞质分裂的分子联系,进一步证明了 Dsk1 对细胞周期进程和调节的多种影响。靶向 MS 分析和体外生化分析确定 Cdr2 蛋白激酶和驱动蛋白运动 Klp9 作为 Dsk1 的新底物,它们分别在有丝分裂进入的细胞大小控制和有丝分裂退出的染色体分离中起作用。由 Dsk1 介导的磷蛋白网络首次揭示了连接 Dsk1 与有丝分裂相变、姐妹染色单体分离和胞质分裂的分子联系,进一步证明了 Dsk1 对细胞周期进程和调节的多种影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号