当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Measurements of Kinetics and Equilibria for the Condensed Phase Reactions of Hydroperoxides with Carbonyls to Form Peroxyhemiacetals
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-02-27 , DOI: 10.1021/acsearthspacechem.0c00008 Julia G. Bakker-Arkema 1, 2 , Paul J. Ziemann 1, 2
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-02-27 , DOI: 10.1021/acsearthspacechem.0c00008 Julia G. Bakker-Arkema 1, 2 , Paul J. Ziemann 1, 2
Affiliation
|
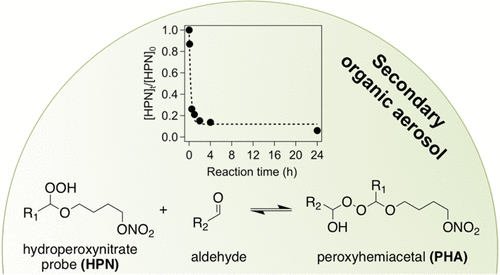
Heterogeneous/multiphase reactions can influence the formation, composition, and chemical–physical properties of secondary organic aerosol (SOA), but data describing their kinetics and equilibria remain sparse. Here, we synthesized and utilized a probe molecule to investigate the condensed phase reactions of hydroperoxides with ketones and aldehydes, including those in SOA generated from the ozonolysis of α-pinene in an environmental chamber. The probe molecule, which contained a hydroperoxide group and a UV-absorbing nitrate group, was mixed with a ketone (3-decanone) or aldehyde (nonanal) and monitored over 24 h using liquid chromatography with UV–vis detection to determine the rate and equilibrium constants for each reaction. The probe molecule did not react with the ketone but reacted reversibly with the aldehyde to form a peroxyhemiacetal, a process that was also catalyzed by carboxylic acid. The rate constant for the reversible decomposition of the peroxyhemiacetal was also measured using attenuated total reflectance Fourier transform infrared spectroscopy. The forward (f) and reverse (r) rate constants for uncatalyzed (u) and catalyzed (c) peroxyhemiacetal formation were kf,u = 1.5 ± 0.4 M–1 h–1, kr,u = 0.16 ± 0.001 h–1, kf,c = 0.62 ± 0.07 M–2 h–1, and kr,c = 0.055 ± 0.006 M–1 h–1; and the equilibrium constant was Keq = 9.1 ± 2 M–1. No evidence of Baeyer–Villiger decomposition of the peroxyhemiacetal was observed. When mixed with α-pinene/O3 SOA, the probe molecule reached reaction equilibrium within 20 min, indicating that atmospheric timescales for peroxyhemiacetal formation can be short. Using the results of the nonanal experiments and measured carbonyl content of the SOA, we estimate that up to 25% of the carbonyls in this SOA was aldehydes.
中文翻译:

氢过氧化物与羰基缩合反应形成过氧半缩醛的动力学和平衡的测量
异相/多相反应会影响次生有机气溶胶(SOA)的形成,组成和化学-物理性质,但是描述其动力学和平衡的数据仍然很少。在这里,我们合成并利用了一个探针分子来研究氢过氧化物与酮和醛的缩合反应,包括在环境室内由α-pine烯的臭氧分解产生的SOA中的缩合反应。将含有氢过氧化物基团和吸收紫外线的硝酸盐基团的探针分子与酮(3-癸酮)或醛(壬醛)混合,并使用液相色谱和紫外线-可见光检测24小时进行监测,以测定速率和每个反应的平衡常数。探针分子不与酮反应,但与醛可逆反应,形成过氧半缩醛,也是由羧酸催化的过程。还使用衰减的全反射傅里叶变换红外光谱法测量过氧半缩醛的可逆分解的速率常数。未催化(u)和催化(c)过氧半缩醛形成的正向(f)和反向(r)速率常数为k f,u = 1.5±0.4 M –1 h –1,k r,u = 0.16±0.001 h –1,k f,c = 0.62±0.07 M –2 h –1,k r,c = 0.055± 0.006 M –1小时–1 ; 平衡常数为K eq = 9.1±2 M –1。没有观察到过氧半缩醛的Baeyer-Villiger分解的证据。与α-pine烯/ O 3混合时SOA,探针分子在20分钟内达到反应平衡,表明过氧半缩醛形成的大气时间尺度可能很短。使用非肛门实验的结果和测得的SOA羰基含量,我们估计该SOA中多达25%的羰基是醛。
更新日期:2020-02-28
中文翻译:

氢过氧化物与羰基缩合反应形成过氧半缩醛的动力学和平衡的测量
异相/多相反应会影响次生有机气溶胶(SOA)的形成,组成和化学-物理性质,但是描述其动力学和平衡的数据仍然很少。在这里,我们合成并利用了一个探针分子来研究氢过氧化物与酮和醛的缩合反应,包括在环境室内由α-pine烯的臭氧分解产生的SOA中的缩合反应。将含有氢过氧化物基团和吸收紫外线的硝酸盐基团的探针分子与酮(3-癸酮)或醛(壬醛)混合,并使用液相色谱和紫外线-可见光检测24小时进行监测,以测定速率和每个反应的平衡常数。探针分子不与酮反应,但与醛可逆反应,形成过氧半缩醛,也是由羧酸催化的过程。还使用衰减的全反射傅里叶变换红外光谱法测量过氧半缩醛的可逆分解的速率常数。未催化(u)和催化(c)过氧半缩醛形成的正向(f)和反向(r)速率常数为k f,u = 1.5±0.4 M –1 h –1,k r,u = 0.16±0.001 h –1,k f,c = 0.62±0.07 M –2 h –1,k r,c = 0.055± 0.006 M –1小时–1 ; 平衡常数为K eq = 9.1±2 M –1。没有观察到过氧半缩醛的Baeyer-Villiger分解的证据。与α-pine烯/ O 3混合时SOA,探针分子在20分钟内达到反应平衡,表明过氧半缩醛形成的大气时间尺度可能很短。使用非肛门实验的结果和测得的SOA羰基含量,我们估计该SOA中多达25%的羰基是醛。

























 京公网安备 11010802027423号
京公网安备 11010802027423号