当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Novel Amino-Functionalized Ionic Liquid/Organic Solvent with Low Viscosity for CO2 Capture.
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-02-16 , DOI: 10.1021/acs.est.9b06717 Fan Liu 1 , Yao Shen 1 , Li Shen 1 , Cheng Sun 1 , Liang Chen 1 , Qiaoli Wang 1 , Sujing Li 1 , Wei Li 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-02-16 , DOI: 10.1021/acs.est.9b06717 Fan Liu 1 , Yao Shen 1 , Li Shen 1 , Cheng Sun 1 , Liang Chen 1 , Qiaoli Wang 1 , Sujing Li 1 , Wei Li 1
Affiliation
|
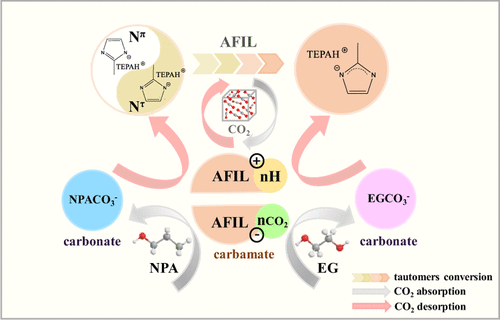
To achieve low regeneration energy consumption and viscosity, a novel amino-functionalized ionic liquid [TEPAH][2-MI] combined with organic solvents has been proposed for CO2 capture in this work. The results demonstrated that the absorption loading of [TEPAH][2-MI]/N-propanol (NPA)/ethylene glycol (EG) was 1.72 mol·mol-1 (28 wt %, 257 g·L-1), which was much higher than that of monoethanolamine/water, and the regeneration efficiency was maintained at 90.7% after the fifth regeneration cycle. The viscosities of the solution were only 3.66 and 7.65 mPa·s before and after absorption, respectively, which were significantly lower than those of traditional nonaqueous absorbents. The reaction mechanism investigated via 13C NMR and quantum calculations summarized that CO2 first reacted with the amino group of [TEPAH]+ to form the carbamates through the zwitterion formation and protonation process, while CO2 reacted with the N atom of [2-MI]- to directly form the carbamate. Then, some of them further reacted with NPA and EG to form the carbonates. Moreover, Nπ and Nτ tautomers of [TEPAH][2-MI] could convert into each other continuously when CO2 was absorbed. During CO2 desorption, the carbamates and carbonates reacted with AFILH+ to decompose and released CO2 directly.
中文翻译:

具有低粘度的新型氨基官能化离子液体/有机溶剂,用于捕集二氧化碳。
为了获得较低的再生能量消耗和粘度,已提出了一种新颖的氨基官能化离子液体[TEPAH] [2-MI]与有机溶剂结合用于这项工作中的CO2捕集。结果表明,[TEPAH] [2-MI] / N-丙醇(NPA)/乙二醇(EG)的吸收负荷为1.72 mol·mol-1(28 wt%,257 g·L-1),远高于单乙醇胺/水,并且在第五次再生循环后,再生效率保持在90.7%。溶液在吸收前后的粘度分别仅为3.66和7.65 mPa·s,明显低于传统的非水吸收剂。通过13 C NMR和量子计算研究的反应机理总结为,CO 2首先通过两性离子形成和质子化过程与[TEPAH] +的氨基反应形成氨基甲酸酯,而CO 2与[2-MI]-的N原子反应。直接形成氨基甲酸酯 然后,它们中的一些进一步与NPA和EG反应形成碳酸盐。而且,[TEPAH] [2-MI]的Nπ和Nτ互变异构体在吸收CO2时可以连续相互转化。在CO2解吸过程中,氨基甲酸酯和碳酸盐与AFILH +反应分解并直接释放CO2。[TEPAH] [2-MI]的Nπ和Nτ互变异构体在吸收CO2时可以连续相互转化。在CO2解吸过程中,氨基甲酸酯和碳酸盐与AFILH +反应分解并直接释放CO2。[TEPAH] [2-MI]的Nπ和Nτ互变异构体在吸收CO2时可以连续相互转化。在二氧化碳脱附过程中,氨基甲酸酯和碳酸盐与AFILH +反应分解并直接释放出二氧化碳。
更新日期:2020-02-28
中文翻译:

具有低粘度的新型氨基官能化离子液体/有机溶剂,用于捕集二氧化碳。
为了获得较低的再生能量消耗和粘度,已提出了一种新颖的氨基官能化离子液体[TEPAH] [2-MI]与有机溶剂结合用于这项工作中的CO2捕集。结果表明,[TEPAH] [2-MI] / N-丙醇(NPA)/乙二醇(EG)的吸收负荷为1.72 mol·mol-1(28 wt%,257 g·L-1),远高于单乙醇胺/水,并且在第五次再生循环后,再生效率保持在90.7%。溶液在吸收前后的粘度分别仅为3.66和7.65 mPa·s,明显低于传统的非水吸收剂。通过13 C NMR和量子计算研究的反应机理总结为,CO 2首先通过两性离子形成和质子化过程与[TEPAH] +的氨基反应形成氨基甲酸酯,而CO 2与[2-MI]-的N原子反应。直接形成氨基甲酸酯 然后,它们中的一些进一步与NPA和EG反应形成碳酸盐。而且,[TEPAH] [2-MI]的Nπ和Nτ互变异构体在吸收CO2时可以连续相互转化。在CO2解吸过程中,氨基甲酸酯和碳酸盐与AFILH +反应分解并直接释放CO2。[TEPAH] [2-MI]的Nπ和Nτ互变异构体在吸收CO2时可以连续相互转化。在CO2解吸过程中,氨基甲酸酯和碳酸盐与AFILH +反应分解并直接释放CO2。[TEPAH] [2-MI]的Nπ和Nτ互变异构体在吸收CO2时可以连续相互转化。在二氧化碳脱附过程中,氨基甲酸酯和碳酸盐与AFILH +反应分解并直接释放出二氧化碳。


























 京公网安备 11010802027423号
京公网安备 11010802027423号