当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemoenzymatic Semisynthesis of Phosphorylated α-Synuclein Enables Identification of a Bidirectional Effect on Fibril Formation.
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-02-17 , DOI: 10.1021/acschembio.9b01038 Buyan Pan 1 , Elizabeth Rhoades 1 , E James Petersson 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-02-17 , DOI: 10.1021/acschembio.9b01038 Buyan Pan 1 , Elizabeth Rhoades 1 , E James Petersson 1
Affiliation
|
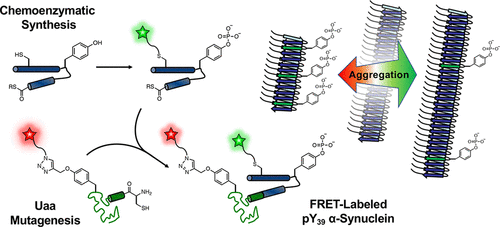
Post-translational modifications (PTMs) impact the pathological aggregation of α-synuclein (αS), a hallmark of Parkinson's disease (PD). Here, we synthesize αS phosphorylated at tyrosine 39 (pY39) through a novel route using in vitro enzymatic phosphorylation of a fragment followed by ligation to form the full-length protein. We can execute this synthesis in combination with unnatural amino acid mutagenesis to include two fluorescent labels for Förster resonance energy transfer (FRET) studies. We determine the effect of pY39 on the aggregation of αS and compare our authentically phosphorylated material to the corresponding glutamate 39 "phosphomimetic." Intriguingly, we find that αS-pY39 can either accelerate or decelerate aggregation, depending on the fraction of phosphorylated protein. The αS-E39 mutant can qualitatively reproduce some, but not all, of these effects. FRET measurements and analysis of existing structures of αS help to provide an explanation for this phenomenon. Our results have important implications for the treatment of PD patients with tyrosine kinase inhibitors and highlight the importance of validating phosphomimetics through studies of authentic PTMs.
中文翻译:

磷酸化α-突触核蛋白的化学酶半合成能够鉴定对原纤维形成的双向作用。
翻译后修饰(PTM)影响帕金森氏病(PD)的标志性α-突触核蛋白(αS)的病理聚集。在这里,我们使用片段的体外酶促磷酸化,然后通过连接形成全长蛋白,通过一条新途径合成了在酪氨酸39(pY39)处磷酸化的αS。我们可以结合非天然氨基酸诱变来执行此合成,以包括两个用于Förster共振能量转移(FRET)研究的荧光标记。我们确定了pY39对αS聚集的影响,并将我们真实地磷酸化的物质与相应的谷氨酸39“拟磷酸酯”进行了比较。有趣的是,我们发现αS-pY39可以加速或减速聚集,具体取决于磷酸化蛋白的比例。αS-E39突变体可以定性地复制其中的部分效应,但并非全部。FRET测量和对现有αS结构的分析有助于对此现象做出解释。我们的结果对于用酪氨酸激酶抑制剂治疗PD患者具有重要意义,并强调了通过对真实PTM进行研究验证仿生药物的重要性。
更新日期:2020-02-17
中文翻译:

磷酸化α-突触核蛋白的化学酶半合成能够鉴定对原纤维形成的双向作用。
翻译后修饰(PTM)影响帕金森氏病(PD)的标志性α-突触核蛋白(αS)的病理聚集。在这里,我们使用片段的体外酶促磷酸化,然后通过连接形成全长蛋白,通过一条新途径合成了在酪氨酸39(pY39)处磷酸化的αS。我们可以结合非天然氨基酸诱变来执行此合成,以包括两个用于Förster共振能量转移(FRET)研究的荧光标记。我们确定了pY39对αS聚集的影响,并将我们真实地磷酸化的物质与相应的谷氨酸39“拟磷酸酯”进行了比较。有趣的是,我们发现αS-pY39可以加速或减速聚集,具体取决于磷酸化蛋白的比例。αS-E39突变体可以定性地复制其中的部分效应,但并非全部。FRET测量和对现有αS结构的分析有助于对此现象做出解释。我们的结果对于用酪氨酸激酶抑制剂治疗PD患者具有重要意义,并强调了通过对真实PTM进行研究验证仿生药物的重要性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号