Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthetic Studies Towards Spirocyclic Imine Marine Toxins Using N-Acyl Iminium Ions as Dienophiles in Diels–Alder Reactions
Synlett ( IF 2 ) Pub Date : 2020-02-13 , DOI: 10.1055/s-0039-1691593 Jared L. Freeman 1 , Freda F. Li 1 , Daniel P. Furkert 1, 2 , Margaret A. Brimble 1, 2
Synlett ( IF 2 ) Pub Date : 2020-02-13 , DOI: 10.1055/s-0039-1691593 Jared L. Freeman 1 , Freda F. Li 1 , Daniel P. Furkert 1, 2 , Margaret A. Brimble 1, 2
Affiliation
|
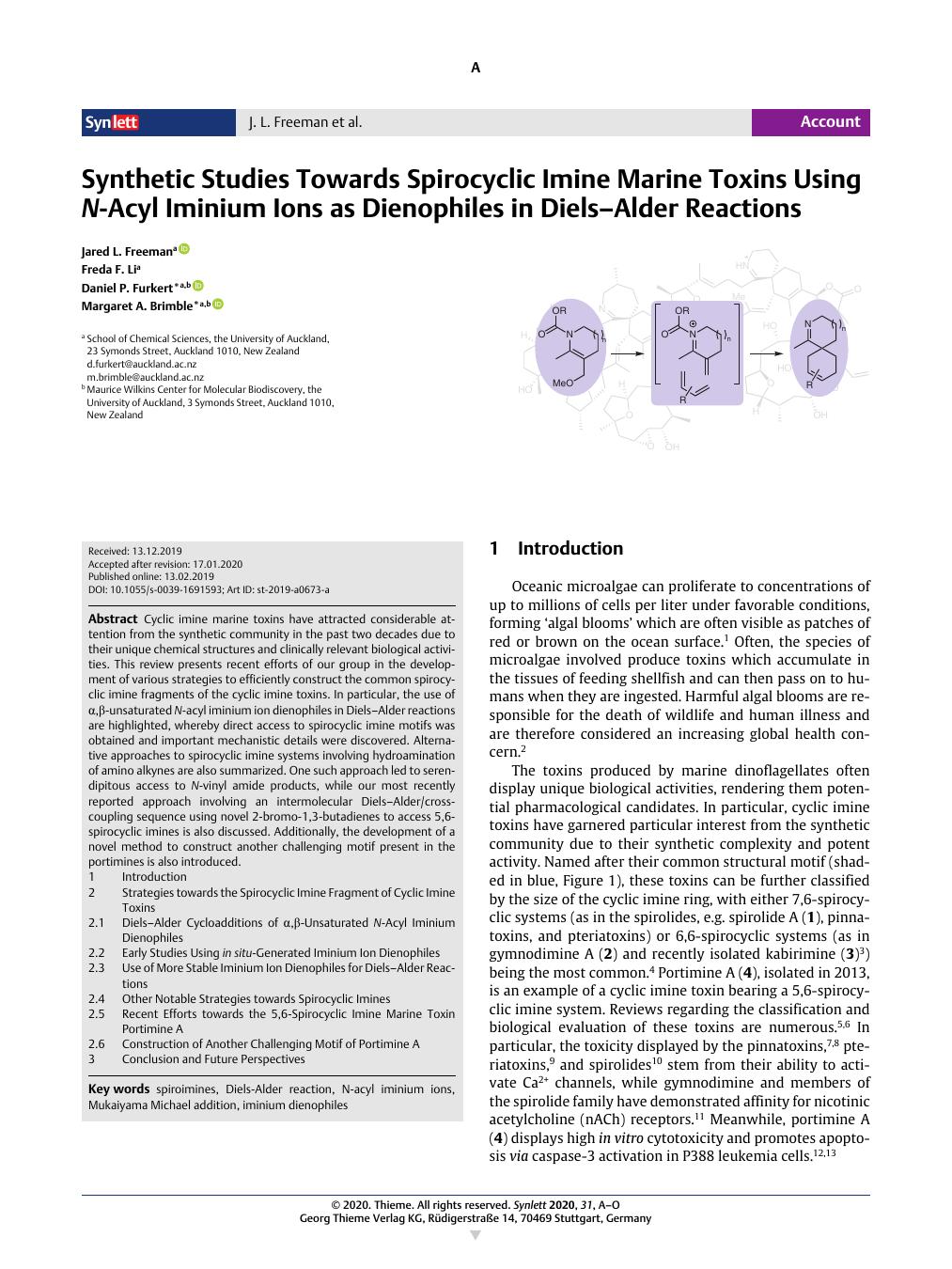
Cyclic imine marine toxins have attracted considerable attention from the synthetic community in the past two decades due to their unique chemical structures and clinically relevant biological activities. This review presents recent efforts of our group in the development of various strategies to efficiently construct the common spirocyclic imine fragments of the cyclic imine toxins. In particular, the use of α,β-unsaturated N-acyl iminium ion dienophiles in Diels–Alder reactions are highlighted, whereby direct access to spirocyclic imine motifs was obtained and important mechanistic details were discovered. Alternative approaches to spirocyclic imine systems involving hydroamination of amino alkynes are also summarized. One such approach led to serendipitous access to N-vinyl amide products, while our most recently reported approach involving an intermolecular Diels–Alder/cross-coupling sequence using novel 2-bromo-1,3-butadienes to access 5,6-spirocyclic imines is also discussed. Additionally, the development of a novel method to construct another challenging motif present in the portimines is also introduced. 1 Introduction 2 Strategies towards the Spirocyclic Imine Fragment of Cyclic Imine Toxins 2.1 Diels–Alder Cycloadditions of α,β-Unsaturated N-Acyl Iminium Dienophiles 2.2 Early Studies Using in situ-Generated Iminium Ion Dienophiles 2.3 Use of More Stable Iminium Ion Dienophiles for Diels–Alder Reactions 2.4 Other Notable Strategies towards Spirocyclic Imines 2.5 Recent Efforts towards the 5,6-Spirocyclic Imine Marine Toxin Portimine A 2.6 Construction of Another Challenging Motif of Portimine A 3 Conclusion and Future Perspectives
中文翻译:

使用 N-酰基亚胺离子作为 Diels-Alder 反应中的亲二烯体对螺环亚胺海洋毒素的合成研究
环亚胺海洋毒素由于其独特的化学结构和临床相关的生物活性,在过去二十年中引起了合成界的广泛关注。这篇综述介绍了我们小组最近在开发各种策略以有效构建环状亚胺毒素的常见螺环亚胺片段方面所做的努力。特别是,强调了在 Diels-Alder 反应中使用 α,β-不饱和 N-酰基亚胺离子亲二烯体,从而获得了对螺环亚胺基序的直接访问,并发现了重要的机制细节。还总结了涉及氨基炔烃加氢胺化的螺环亚胺系统的替代方法。其中一种方法导致意外获得 N-乙烯基酰胺产品,同时还讨论了我们最近报道的涉及分子间 Diels-Alder/交叉偶联序列的方法,使用新型 2-溴-1,3-丁二烯来获得 5,6-螺环亚胺。此外,还介绍了构建门廊中存在的另一个具有挑战性的主题的新方法的开发。1 介绍 2 环亚胺毒素的螺环亚胺片段的策略 2.1 α,β-不饱和 N-酰基亚胺亲二烯体的 Diels-Alder 环加成 2.2 使用原位生成的亚胺离子亲二烯体的早期研究 2.3 使用更稳定的二烯二烯体– Alder 反应 2.4 其他显着的螺环亚胺策略 2.5 最近对 5,6-螺环亚胺海洋毒素 Portimine A 2 的努力。
更新日期:2020-02-13
中文翻译:

使用 N-酰基亚胺离子作为 Diels-Alder 反应中的亲二烯体对螺环亚胺海洋毒素的合成研究
环亚胺海洋毒素由于其独特的化学结构和临床相关的生物活性,在过去二十年中引起了合成界的广泛关注。这篇综述介绍了我们小组最近在开发各种策略以有效构建环状亚胺毒素的常见螺环亚胺片段方面所做的努力。特别是,强调了在 Diels-Alder 反应中使用 α,β-不饱和 N-酰基亚胺离子亲二烯体,从而获得了对螺环亚胺基序的直接访问,并发现了重要的机制细节。还总结了涉及氨基炔烃加氢胺化的螺环亚胺系统的替代方法。其中一种方法导致意外获得 N-乙烯基酰胺产品,同时还讨论了我们最近报道的涉及分子间 Diels-Alder/交叉偶联序列的方法,使用新型 2-溴-1,3-丁二烯来获得 5,6-螺环亚胺。此外,还介绍了构建门廊中存在的另一个具有挑战性的主题的新方法的开发。1 介绍 2 环亚胺毒素的螺环亚胺片段的策略 2.1 α,β-不饱和 N-酰基亚胺亲二烯体的 Diels-Alder 环加成 2.2 使用原位生成的亚胺离子亲二烯体的早期研究 2.3 使用更稳定的二烯二烯体– Alder 反应 2.4 其他显着的螺环亚胺策略 2.5 最近对 5,6-螺环亚胺海洋毒素 Portimine A 2 的努力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号