当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Charge transfer as a ubiquitous mechanism in determining the negative charge at hydrophobic interfaces.
Nature Communications ( IF 16.6 ) Pub Date : 2020-02-14 , DOI: 10.1038/s41467-020-14659-5 Emiliano Poli 1 , Kwang H Jong 1 , Ali Hassanali 1
Nature Communications ( IF 16.6 ) Pub Date : 2020-02-14 , DOI: 10.1038/s41467-020-14659-5 Emiliano Poli 1 , Kwang H Jong 1 , Ali Hassanali 1
Affiliation
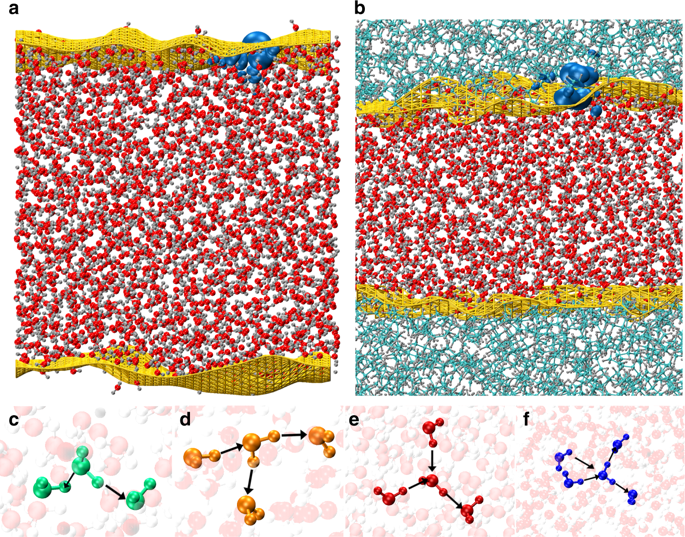
|
The origin of the apparent negative charge at hydrophobic-water interfaces has fueled debates in the physical chemistry community for decades. The most common interpretation given to explain this observation is that negatively charged hydroxide ions (OH-) bind strongly to the interfaces. Using first principles calculations of extended air-water and oil-water interfaces, we unravel a mechanism that does not require the presence of OH-. Small amounts of charge transfer along hydrogen bonds and asymmetries in the hydrogen bond network due to topological defects can lead to the accumulation of negative surface charge at both interfaces. For water near oil, some spillage of electron density into the oil phase is also observed. The computed surface charge densities at both interfaces is approximately [Formula: see text] in agreement with electrophoretic experiments. We also show, using an energy decomposition analysis, that the electronic origin of this phenomena is rooted in a collective polarization/charge transfer effect.
中文翻译:

电荷转移是确定疏水界面负电荷的普遍机制。
数十年来,疏水-水界面上明显的负电荷的起源激起了物理化学界的争论。用来解释该现象的最常见解释是带负电荷的氢氧根离子(OH-)与界面牢固结合。使用扩展空气-水和油-水界面的第一性原理计算,我们揭示了一种不需要OH-的机理。由于拓扑缺陷,沿着氢键和氢键网络中的不对称性沿着氢键的少量电荷转移会导致两个界面处的负表面电荷积聚。对于油附近的水,也观察到一些电子密度溢出到油相中。在两个界面上计算的表面电荷密度约为[公式:见文字]与电泳实验一致。我们还使用能量分解分析表明,这种现象的电子起源根源于集体极化/电荷转移效应。
更新日期:2020-02-14
中文翻译:

电荷转移是确定疏水界面负电荷的普遍机制。
数十年来,疏水-水界面上明显的负电荷的起源激起了物理化学界的争论。用来解释该现象的最常见解释是带负电荷的氢氧根离子(OH-)与界面牢固结合。使用扩展空气-水和油-水界面的第一性原理计算,我们揭示了一种不需要OH-的机理。由于拓扑缺陷,沿着氢键和氢键网络中的不对称性沿着氢键的少量电荷转移会导致两个界面处的负表面电荷积聚。对于油附近的水,也观察到一些电子密度溢出到油相中。在两个界面上计算的表面电荷密度约为[公式:见文字]与电泳实验一致。我们还使用能量分解分析表明,这种现象的电子起源根源于集体极化/电荷转移效应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号