当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Controlled depolymerization of cellulose by light-driven lytic polysaccharide oxygenases.
Nature Communications ( IF 16.6 ) Pub Date : 2020-02-14 , DOI: 10.1038/s41467-020-14744-9 Bastien Bissaro 1, 2 , Eirik Kommedal 1 , Åsmund K Røhr 1 , Vincent G H Eijsink 1
Nature Communications ( IF 16.6 ) Pub Date : 2020-02-14 , DOI: 10.1038/s41467-020-14744-9 Bastien Bissaro 1, 2 , Eirik Kommedal 1 , Åsmund K Røhr 1 , Vincent G H Eijsink 1
Affiliation
|
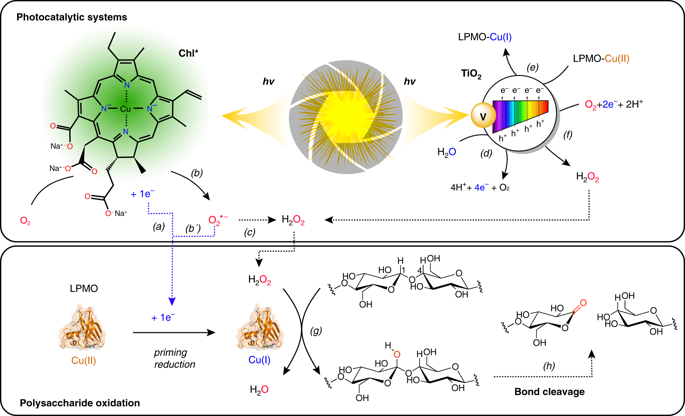
Lytic polysaccharide (mono)oxygenases (LPMOs) perform oxidative cleavage of polysaccharides, and are key enzymes in biomass processing and the global carbon cycle. It has been shown that LPMO reactions may be driven by light, using photosynthetic pigments or photocatalysts, but the mechanism behind this highly attractive catalytic route remains unknown. Here, prompted by the discovery that LPMOs catalyze a peroxygenase reaction more efficiently than a monooxygenase reaction, we revisit these light-driven systems, using an LPMO from Streptomyces coelicolor (ScAA10C) as model cellulolytic enzyme. By using coupled enzymatic assays, we show that H2O2 is produced and necessary for efficient light-driven activity of ScAA10C. Importantly, this activity is achieved without addition of reducing agents and proportional to the light intensity. Overall, the results highlight the importance of controlling fluxes of reactive oxygen species in LPMO reactions and demonstrate the feasibility of light-driven, tunable enzymatic peroxygenation to degrade recalcitrant polysaccharides.
中文翻译:

通过光驱动裂解多糖加氧酶控制纤维素的解聚。
溶菌多糖(单)加氧酶(LPMO)执行多糖的氧化裂解,是生物质加工和全球碳循环中的关键酶。已经表明,使用光合颜料或光催化剂可以通过光驱动LPMO反应,但是这种高度吸引人的催化途径背后的机理仍然未知。在这里,由于发现LPMO比单加氧酶反应更有效地催化了过氧化酶反应,我们使用产自链霉菌(Streptomyces coelicolor)的LPMO(ScAA10C)作为模型纤维素分解酶来重新研究这些光驱动系统。通过使用耦合的酶法测定,我们表明产生了H2O2,它是ScAA10C的有效光驱动活性所必需的。重要的是,无需添加还原剂且与光强度成比例即可实现该活性。总体,
更新日期:2020-02-14
中文翻译:

通过光驱动裂解多糖加氧酶控制纤维素的解聚。
溶菌多糖(单)加氧酶(LPMO)执行多糖的氧化裂解,是生物质加工和全球碳循环中的关键酶。已经表明,使用光合颜料或光催化剂可以通过光驱动LPMO反应,但是这种高度吸引人的催化途径背后的机理仍然未知。在这里,由于发现LPMO比单加氧酶反应更有效地催化了过氧化酶反应,我们使用产自链霉菌(Streptomyces coelicolor)的LPMO(ScAA10C)作为模型纤维素分解酶来重新研究这些光驱动系统。通过使用耦合的酶法测定,我们表明产生了H2O2,它是ScAA10C的有效光驱动活性所必需的。重要的是,无需添加还原剂且与光强度成比例即可实现该活性。总体,



























 京公网安备 11010802027423号
京公网安备 11010802027423号