当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Antiepileptic Drug Carbamazepine Binds to a Novel Pocket on the Wnt Receptor Frizzled-8.
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-02-25 , DOI: 10.1021/acs.jmedchem.9b02020 Yuguang Zhao 1 , Jingshan Ren 1 , James Hillier 1 , Weixian Lu 1 , E Yvonne Jones 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-02-25 , DOI: 10.1021/acs.jmedchem.9b02020 Yuguang Zhao 1 , Jingshan Ren 1 , James Hillier 1 , Weixian Lu 1 , E Yvonne Jones 1
Affiliation
|
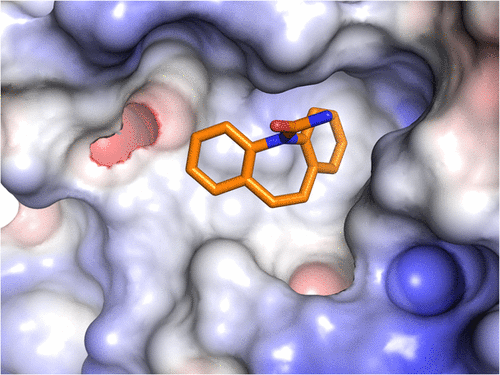
Misregulation of Wnt signaling is common in human cancer. The development of small molecule inhibitors against the Wnt receptor, frizzled (FZD), may have potential in cancer therapy. During small molecule screens, we observed binding of carbamazepine to the cysteine-rich domain (CRD) of the Wnt receptor FZD8 using surface plasmon resonance (SPR). Cellular functional assays demonstrated that carbamazepine can suppress FZD8-mediated Wnt/β-catenin signaling. We determined the crystal structure of the complex at 1.7 Å resolution, which reveals that carbamazepine binds at a novel pocket on the FZD8 CRD. The unique residue Tyr52 discriminates FZD8 from the closely related FZD5 and other FZDs for carbamazepine binding. The first small molecule-bound FZD structure provides a basis for anti-FZD drug development. Furthermore, the observed carbamazepine-mediated Wnt signaling inhibition may help to explain the phenomenon of bone loss and increased adipogenesis in some patients during long-term carbamazepine treatment.
中文翻译:

抗癫痫药物卡马西平与Wnt受体Frizzled-8的新型口袋结合。
Wnt信号的失调在人类癌症中很常见。抗Wnt受体的小分子抑制剂(FZD)的开发可能在癌症治疗中具有潜力。在小分子筛选过程中,我们观察到卡马西平与Wnt受体FZD8的富含半胱氨酸的结构域(CRD)的结合,包括表面等离子体共振(SPR)。细胞功能测定表明,卡马西平可以抑制FZD8介导的Wnt /β-catenin信号传导。我们以1.7Å的分辨率确定了该复合物的晶体结构,这表明卡马西平在FZD8 CRD的一个新口袋处结合。独特的Tyr52残基将FZD8与密切相关的FZD5和其他FZD区分为具有卡马西平。第一个与小分子结合的FZD结构为抗FZD药物开发提供了基础。此外,
更新日期:2020-02-25
中文翻译:

抗癫痫药物卡马西平与Wnt受体Frizzled-8的新型口袋结合。
Wnt信号的失调在人类癌症中很常见。抗Wnt受体的小分子抑制剂(FZD)的开发可能在癌症治疗中具有潜力。在小分子筛选过程中,我们观察到卡马西平与Wnt受体FZD8的富含半胱氨酸的结构域(CRD)的结合,包括表面等离子体共振(SPR)。细胞功能测定表明,卡马西平可以抑制FZD8介导的Wnt /β-catenin信号传导。我们以1.7Å的分辨率确定了该复合物的晶体结构,这表明卡马西平在FZD8 CRD的一个新口袋处结合。独特的Tyr52残基将FZD8与密切相关的FZD5和其他FZD区分为具有卡马西平。第一个与小分子结合的FZD结构为抗FZD药物开发提供了基础。此外,



























 京公网安备 11010802027423号
京公网安备 11010802027423号