当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phosphorylation of a Disordered Peptide-Structural Effects and Force Field Inconsistencies.
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2020-02-25 , DOI: 10.1021/acs.jctc.9b01190 Ellen Rieloff 1 , Marie Skepö 1, 2
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2020-02-25 , DOI: 10.1021/acs.jctc.9b01190 Ellen Rieloff 1 , Marie Skepö 1, 2
Affiliation
|
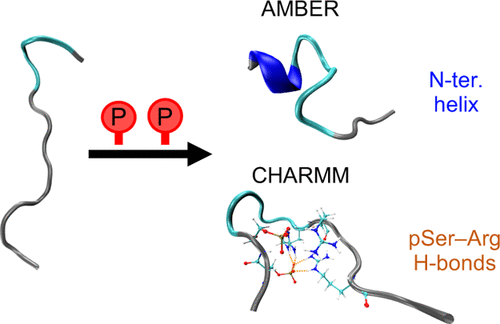
Phosphorylation is one of the most abundant types of post-translational modifications of intrinsically disordered proteins (IDPs). This study examines the conformational changes in the 15-residue-long N-terminal fragment of the IDP statherin upon phosphorylation, using computer simulations with two different force fields: AMBER ff99SB-ILDN and CHARMM36m. The results from the simulations are compared with experimental small-angle X-ray scattering (SAXS) and circular dichroism data. In the unphosphorylated state, the two force fields are in excellent agreement regarding global structural properties such as size and shape. However, they exhibit some differences in the extent and type of the secondary structure. In the phosphorylated state, neither of the force fields performs well compared to the experimental data. Both force fields show a compaction of the peptide upon phosphorylation, greater than what is seen in SAXS experiments, although they differ in the local structure. While the CHARMM force field increases the fraction of bends in the peptide as a response to strong interactions between the phosphorylated residues and arginines, the AMBER force field shows an increase of the helical content in the N-terminal part of the peptide, where the phosphorylated residues reside, in better agreement with circular dichroism results.
中文翻译:

磷酸化的无序肽结构效应和力场不一致。
磷酸化是内在无序蛋白(IDP)的翻译后修饰的最丰富类型之一。这项研究使用两个不同的力场:AMBER ff99SB-ILDN和CHARMM36m,通过计算机模拟研究了IDP伐他汀的15个残基长的N末端片段在磷酸化后的构象变化。将模拟结果与实验性小角度X射线散射(SAXS)和圆二色性数据进行比较。在未磷酸化的状态下,两个力场在整体结构特性(例如大小和形状)方面非常一致。但是,它们在二级结构的范围和类型上表现出一些差异。与实验数据相比,在磷酸化状态下,两个力场都不能很好地发挥作用。尽管两个力场在局部结构上有所不同,但它们在磷酸化后均显示出肽的紧实度,大于在SAXS实验中看到的紧密度。尽管CHARMM力场响应于磷酸化残基和精氨酸之间的强相互作用而增加了肽的弯曲比例,但AMBER力场显示出了肽N端部分中的螺旋含量增加了,其中磷酸化了残留物与圆形二色性结果更好地吻合。
更新日期:2020-02-25
中文翻译:

磷酸化的无序肽结构效应和力场不一致。
磷酸化是内在无序蛋白(IDP)的翻译后修饰的最丰富类型之一。这项研究使用两个不同的力场:AMBER ff99SB-ILDN和CHARMM36m,通过计算机模拟研究了IDP伐他汀的15个残基长的N末端片段在磷酸化后的构象变化。将模拟结果与实验性小角度X射线散射(SAXS)和圆二色性数据进行比较。在未磷酸化的状态下,两个力场在整体结构特性(例如大小和形状)方面非常一致。但是,它们在二级结构的范围和类型上表现出一些差异。与实验数据相比,在磷酸化状态下,两个力场都不能很好地发挥作用。尽管两个力场在局部结构上有所不同,但它们在磷酸化后均显示出肽的紧实度,大于在SAXS实验中看到的紧密度。尽管CHARMM力场响应于磷酸化残基和精氨酸之间的强相互作用而增加了肽的弯曲比例,但AMBER力场显示出了肽N端部分中的螺旋含量增加了,其中磷酸化了残留物与圆形二色性结果更好地吻合。


























 京公网安备 11010802027423号
京公网安备 11010802027423号