当前位置:
X-MOL 学术
›
Nat. Rev. Clin. Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Immunotherapeutic approaches for small-cell lung cancer.
Nature Reviews Clinical Oncology ( IF 78.8 ) Pub Date : 2020-02-13 , DOI: 10.1038/s41571-019-0316-z Wade T Iams 1 , Jason Porter 2 , Leora Horn 1
Nature Reviews Clinical Oncology ( IF 78.8 ) Pub Date : 2020-02-13 , DOI: 10.1038/s41571-019-0316-z Wade T Iams 1 , Jason Porter 2 , Leora Horn 1
Affiliation
|
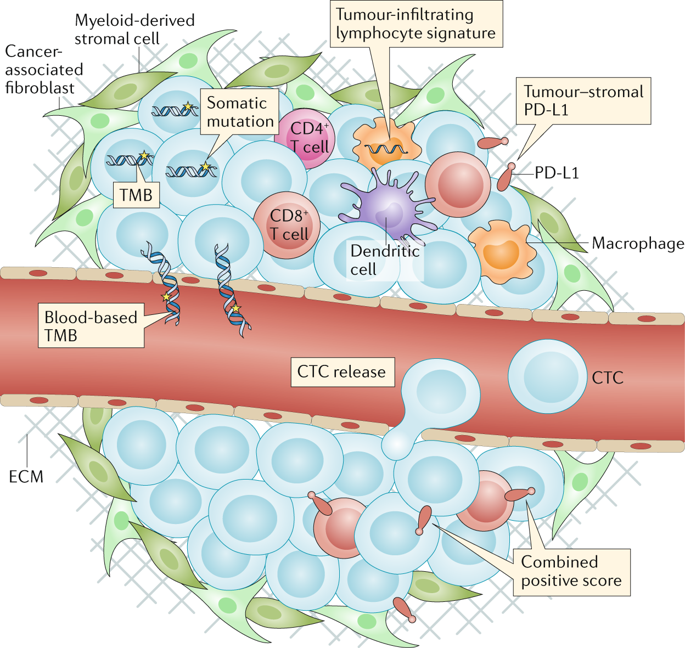
Immune-checkpoint inhibitors (ICIs) are approved in the first-line and third-line settings for patients with extensive-stage or relapsed small-cell lung cancer (SCLC), respectively. In the first-line setting, the addition of the anti-programmed cell death 1 ligand 1 (PD-L1) antibody atezolizumab to chemotherapy improves overall survival (OS). In patients with relapsed disease, data from nonrandomized trials have revealed promising responses, although a significant improvement in OS over that obtained with conventional chemotherapy was not achieved in a randomized trial in this setting. Substantial research interest exists in identifying predictive biomarkers that could guide the use of ICIs in patients with SCLC. PD-L1 expression is typically low or absent in SCLC, which has precluded its use as a predictive biomarker. Tumour mutational burden might have some predictive value, although blood-based measures of tumour mutational burden did not have predictive value in patients receiving atezolizumab plus chemotherapy in the first-line setting. After three decades, ICIs have finally enabled an improvement in OS for patients with SCLC; however, a substantial amount of research remains to be done, including identifying the optimal therapeutic strategy and predictive biomarkers. In this Review, we describe the available data on clinical efficacy, the emerging evidence regarding biomarkers and ongoing clinical trials using ICIs and other immunotherapies in patients with SCLC.
中文翻译:

小细胞肺癌的免疫治疗方法。
一线和三线环境中分别批准了免疫检查点抑制剂(ICIs)用于患有广泛期或复发性小细胞肺癌(SCLC)的患者。在第一线治疗中,将抗程序性细胞死亡1配体1(PD-L1)抗体atezolizumab加入化疗可提高总体生存率(OS)。在复发性疾病患者中,来自非随机试验的数据显示出令人鼓舞的反应,尽管在这种情况下的一项随机试验中,与传统化学疗法相比,OS并未获得明显改善。存在大量的研究兴趣来确定可指导SCLC患者ICI使用的预测性生物标志物。PD-L1表达在SCLC中通常较低或不存在,这使其无法用作预测性生物标志物。肿瘤突变负荷可能具有一定的预测价值,尽管在一线治疗中接受atezolizumab联合化疗的患者的基于血液的肿瘤突变负荷测量没有预测价值。三十年后,ICI终于使SCLC患者的OS得以改善。然而,仍然有大量的研究要做,包括确定最佳的治疗策略和预测性生物标志物。在本综述中,我们描述了有关SCLC患者的临床疗效,有关生物标志物的新兴证据以及正在使用ICI和其他免疫疗法进行的临床试验的可用数据。三十年后,ICI终于使SCLC患者的OS得以改善。然而,仍然有大量的研究要做,包括确定最佳的治疗策略和预测性生物标志物。在本综述中,我们描述了有关SCLC患者的临床疗效,有关生物标志物的新兴证据以及正在使用ICI和其他免疫疗法进行的临床试验的可用数据。三十年后,ICI终于使SCLC患者的OS得以改善。然而,仍然有大量的研究要做,包括确定最佳的治疗策略和预测性生物标志物。在本综述中,我们描述了有关SCLC患者的临床疗效,有关生物标志物的新兴证据以及正在使用ICI和其他免疫疗法进行的临床试验的可用数据。
更新日期:2020-02-13
中文翻译:

小细胞肺癌的免疫治疗方法。
一线和三线环境中分别批准了免疫检查点抑制剂(ICIs)用于患有广泛期或复发性小细胞肺癌(SCLC)的患者。在第一线治疗中,将抗程序性细胞死亡1配体1(PD-L1)抗体atezolizumab加入化疗可提高总体生存率(OS)。在复发性疾病患者中,来自非随机试验的数据显示出令人鼓舞的反应,尽管在这种情况下的一项随机试验中,与传统化学疗法相比,OS并未获得明显改善。存在大量的研究兴趣来确定可指导SCLC患者ICI使用的预测性生物标志物。PD-L1表达在SCLC中通常较低或不存在,这使其无法用作预测性生物标志物。肿瘤突变负荷可能具有一定的预测价值,尽管在一线治疗中接受atezolizumab联合化疗的患者的基于血液的肿瘤突变负荷测量没有预测价值。三十年后,ICI终于使SCLC患者的OS得以改善。然而,仍然有大量的研究要做,包括确定最佳的治疗策略和预测性生物标志物。在本综述中,我们描述了有关SCLC患者的临床疗效,有关生物标志物的新兴证据以及正在使用ICI和其他免疫疗法进行的临床试验的可用数据。三十年后,ICI终于使SCLC患者的OS得以改善。然而,仍然有大量的研究要做,包括确定最佳的治疗策略和预测性生物标志物。在本综述中,我们描述了有关SCLC患者的临床疗效,有关生物标志物的新兴证据以及正在使用ICI和其他免疫疗法进行的临床试验的可用数据。三十年后,ICI终于使SCLC患者的OS得以改善。然而,仍然有大量的研究要做,包括确定最佳的治疗策略和预测性生物标志物。在本综述中,我们描述了有关SCLC患者的临床疗效,有关生物标志物的新兴证据以及正在使用ICI和其他免疫疗法进行的临床试验的可用数据。



























 京公网安备 11010802027423号
京公网安备 11010802027423号