当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
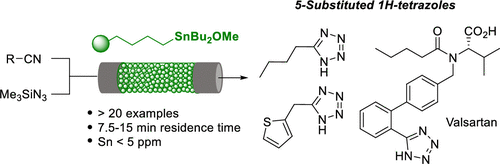
Synthesis of 5-Substituted 1H-Tetrazoles from Nitriles by Continuous Flow: Application to the Synthesis of Valsartan
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-02-10 , DOI: 10.1021/acs.oprd.9b00526 Florian Carpentier 1 , François-Xavier Felpin 1 , Françoise Zammattio 1 , Erwan Le Grognec 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-02-10 , DOI: 10.1021/acs.oprd.9b00526 Florian Carpentier 1 , François-Xavier Felpin 1 , Françoise Zammattio 1 , Erwan Le Grognec 1
Affiliation
|
An efficient continuous flow process for the synthesis of 5-substituted 1H-tetrazoles is described. The process involves the reaction between a polymer-supported triorganotin azide and organic nitriles. The polymer-supported organotin azide, which is in situ generated with a polystyrene-supported triorganotin alkoxide and trimethylsilylazide, is immobilized in a packed bed reactor. This approach is simple, fast (it takes from 7.5 to 15 min), and guarantees a low concentration of tin residues in the products (<5 ppm). The process was developed to aryl-, heteroaryl-, and also alkylnitriles and was applied for the synthesis of valsartan, an angiotensin II receptor antagonist.
中文翻译:

连续流动从腈中合成5-取代的1 H-四唑:在缬沙坦合成中的应用
描述了合成5-取代的1 H-四唑的有效连续流方法。该方法涉及聚合物负载的三有机锡叠氮化物与有机腈之间的反应。用聚苯乙烯负载的三有机锡醇盐和三甲基甲硅烷基叠氮化物原位生成的聚合物负载的有机锡叠氮化物被固定在填充床反应器中。这种方法简单,快速(耗时7.5至15分钟),并保证了产品中锡残留物的浓度低(<5 ppm)。该方法开发为芳基,杂芳基和烷基腈,并用于合成血管紧张素II受体拮抗剂缬沙坦。
更新日期:2020-02-10
中文翻译:

连续流动从腈中合成5-取代的1 H-四唑:在缬沙坦合成中的应用
描述了合成5-取代的1 H-四唑的有效连续流方法。该方法涉及聚合物负载的三有机锡叠氮化物与有机腈之间的反应。用聚苯乙烯负载的三有机锡醇盐和三甲基甲硅烷基叠氮化物原位生成的聚合物负载的有机锡叠氮化物被固定在填充床反应器中。这种方法简单,快速(耗时7.5至15分钟),并保证了产品中锡残留物的浓度低(<5 ppm)。该方法开发为芳基,杂芳基和烷基腈,并用于合成血管紧张素II受体拮抗剂缬沙坦。


























 京公网安备 11010802027423号
京公网安备 11010802027423号