当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
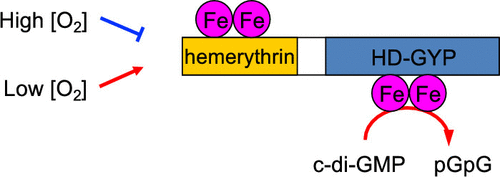
Identification and Characterization of a Redox Sensor Phosphodiesterase from Ferrovum sp. PN-J185 Containing Bacterial Hemerythrin and HD-GYP Domains.
Biochemistry ( IF 2.9 ) Pub Date : 2020-02-20 , DOI: 10.1021/acs.biochem.0c00021 Kenichi Kitanishi 1, 2, 3 , Jotaro Igarashi 4 , Ariki Matsuoka 4 , Masaki Unno 2, 3
Biochemistry ( IF 2.9 ) Pub Date : 2020-02-20 , DOI: 10.1021/acs.biochem.0c00021 Kenichi Kitanishi 1, 2, 3 , Jotaro Igarashi 4 , Ariki Matsuoka 4 , Masaki Unno 2, 3
Affiliation
|
The second messenger bis(3',5')-cyclic dimeric guanosine monophosphate (c-di-GMP) regulates numerous important physiological functions in bacteria. In this study, we identified and characterized the first dimeric, full-length, non-heme iron-bound phosphodiesterase (PDE) containing bacterial hemerythrin and HD-GYP domains (Bhr-HD-GYP). We found that the amino acid sequence encoded by the FV185_09380 gene from Ferrovum sp. PN-J185 contains an N-terminal bacterial hemerythrin domain and a C-terminal HD-GYP domain, which is characteristic of proteins with PDE activity toward c-di-GMP. Inductively coupled plasma optical emission spectroscopy analyses showed that Bhr-HD-GYP contains 4 equiv of iron atoms per subunit, suggesting both hemerythrin and HD-GYP domains have non-heme di-iron sites. A redox-dependent spectral change expected for oxo-bridged non-heme iron with carboxylate ligands was observed, and this redox interconversion was reversible. However, unlike marine invertebrate hemerythrin, which functions as an oxygen-binding protein, Bhr-HD-GYP did not form an oxygen adduct because of rapid autoxidation. The reduced ferrous iron complex of the protein catalyzed the hydrolysis of c-di-GMP to its linearized product, 5'-phosphoguanylyl-(3',5')-guanosine (pGpG), whereas the oxidized ferric iron complex had no significant activity. These results suggest that Bhr-HD-GYP is a redox and oxygen sensor enzyme that regulates c-di-GMP levels in response to changes in cellular redox status or oxygen concentration. Our study may lead to an improved understanding of the physiology of iron-oxidizing bacterium Ferrovum sp. PN-J185.
中文翻译:

鉴定和表征的氧化还原传感器磷酸二酯酶。PN-J185含有细菌性红杉素和HD-GYP域。
第二信使双(3',5')-环二聚鸟苷单磷酸(c-di-GMP)调节细菌中许多重要的生理功能。在这项研究中,我们鉴定并鉴定了第一个包含细菌性血红蛋白和HD-GYP结构域(Bhr-HD-GYP)的全长二聚体,非血红素铁结合磷酸二酯酶(PDE)。我们发现由Ferrovum sp。的FV185_09380基因编码的氨基酸序列。PN-J185包含一个N端细菌半胱氨酸结构域和一个C端HD-GYP结构域,这是具有对c-di-GMP的PDE活性的蛋白质的特征。电感耦合等离子体发射光谱分析表明,Bhr-HD-GYP每个亚基包含4个当量的铁原子,表明苏木精和HD-GYP域均具有非血红素二铁位点。观察到具有羧酸根配体的氧桥非血红素铁预期的氧化还原依赖性光谱变化,并且该氧化还原互变是可逆的。但是,与作为无氧结合蛋白的海洋无脊椎动物苏木精不同,由于快速的自氧化作用,Bhr-HD-GYP不会形成氧加合物。蛋白质的还原亚铁络合物催化c-di-GMP水解为其线性化产物5'-磷酸鸟苷基-(3',5')-鸟苷(pGpG),而氧化的三价铁络合物则无明显活性。这些结果表明,Bhr-HD-GYP是一种氧化还原和氧传感器酶,响应于细胞氧化还原状态或氧气浓度的变化而调节c-di-GMP的水平。我们的研究可能会导致对铁氧化细菌Ferrovum sp。的生理学的更好理解。PN-J185。
更新日期:2020-02-21
中文翻译:

鉴定和表征的氧化还原传感器磷酸二酯酶。PN-J185含有细菌性红杉素和HD-GYP域。
第二信使双(3',5')-环二聚鸟苷单磷酸(c-di-GMP)调节细菌中许多重要的生理功能。在这项研究中,我们鉴定并鉴定了第一个包含细菌性血红蛋白和HD-GYP结构域(Bhr-HD-GYP)的全长二聚体,非血红素铁结合磷酸二酯酶(PDE)。我们发现由Ferrovum sp。的FV185_09380基因编码的氨基酸序列。PN-J185包含一个N端细菌半胱氨酸结构域和一个C端HD-GYP结构域,这是具有对c-di-GMP的PDE活性的蛋白质的特征。电感耦合等离子体发射光谱分析表明,Bhr-HD-GYP每个亚基包含4个当量的铁原子,表明苏木精和HD-GYP域均具有非血红素二铁位点。观察到具有羧酸根配体的氧桥非血红素铁预期的氧化还原依赖性光谱变化,并且该氧化还原互变是可逆的。但是,与作为无氧结合蛋白的海洋无脊椎动物苏木精不同,由于快速的自氧化作用,Bhr-HD-GYP不会形成氧加合物。蛋白质的还原亚铁络合物催化c-di-GMP水解为其线性化产物5'-磷酸鸟苷基-(3',5')-鸟苷(pGpG),而氧化的三价铁络合物则无明显活性。这些结果表明,Bhr-HD-GYP是一种氧化还原和氧传感器酶,响应于细胞氧化还原状态或氧气浓度的变化而调节c-di-GMP的水平。我们的研究可能会导致对铁氧化细菌Ferrovum sp。的生理学的更好理解。PN-J185。


























 京公网安备 11010802027423号
京公网安备 11010802027423号