当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Studies of Bioorthogonal ATP Analogues for Assessment of Histidine Kinase Autophosphorylation.
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-02-24 , DOI: 10.1021/acschembio.9b01024 Adeline Espinasse 1 , Xuelan Wen 1 , Jason D Goodpaster 1 , Erin E Carlson 1, 2, 3
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-02-24 , DOI: 10.1021/acschembio.9b01024 Adeline Espinasse 1 , Xuelan Wen 1 , Jason D Goodpaster 1 , Erin E Carlson 1, 2, 3
Affiliation
|
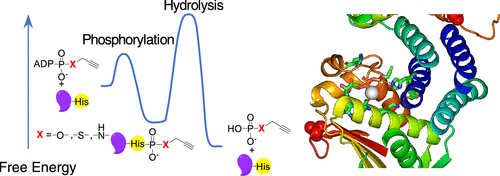
Phosphorylation is an essential protein modification and is most commonly associated with hydroxyl-containing amino acids via an adenosine triphosphate (ATP) substrate. The last decades have brought greater appreciation to the roles that phosphorylation of myriad amino acids plays in biological signaling, metabolism, and gene transcription. Histidine phosphorylation occurs in both eukaryotes and prokaryotes but has been shown to dominate signaling networks in the latter due to its role in microbial two-component systems. Methods to investigate histidine phosphorylation have lagged behind those to study serine, threonine, and tyrosine modifications due to its inherent instability and the historical view that this protein modification was rare. An important strategy to overcome the reactivity of phosphohistidine is the development of substrate-based probes with altered chemical properties that improve modification longevity but that do not suffer from poor recognition or transfer by the protein. Here, we present combined experimental and computational studies to better understand the molecular requirements for efficient histidine phosphorylation by comparison of the native kinase substrate, ATP, and alkylated ATP derivatives. While recognition of the substrates by the histidine kinases is an important parameter for the formation of phosphohistidine derivatives, reaction sterics also affect the outcome. In addition, we found that stability of the resulting phosphohistidine moieties correlates with the stability of their hydrolysis products, specifically with their free energy in solution. Interestingly, alkylation dramatically affects the stability of the phosphohistidine derivatives at very acidic pH values. These results provide critical mechanistic insights into histidine phosphorylation and will facilitate the design of future probes to study enzymatic histidine phosphorylation.
中文翻译:

生物正交 ATP 类似物用于评估组氨酸激酶自磷酸化的机制研究。
磷酸化是一种必不可少的蛋白质修饰,最常见的是通过三磷酸腺苷 (ATP) 底物与含羟基的氨基酸相关联。在过去的几十年里,人们对无数氨基酸的磷酸化在生物信号、代谢和基因转录中所起的作用有了更大的认识。组氨酸磷酸化发生在真核生物和原核生物中,但由于其在微生物双组分系统中的作用,已被证明在后者中占主导地位。由于其固有的不稳定性以及这种蛋白质修饰很少见的历史观点,研究组氨酸磷酸化的方法落后于研究丝氨酸、苏氨酸和酪氨酸修饰的方法。克服磷酸组氨酸反应性的一个重要策略是开发具有改变化学性质的基于底物的探针,以提高修饰寿命,但不会受到蛋白质识别或转移的影响。在这里,我们结合实验和计算研究,通过比较天然激酶底物、ATP 和烷基化 ATP 衍生物,更好地了解有效组氨酸磷酸化的分子要求。虽然组氨酸激酶对底物的识别是形成磷酸组氨酸衍生物的重要参数,但反应空间也影响结果。此外,我们发现所得磷酸组氨酸部分的稳定性与其水解产物的稳定性相关,特别是与它们在溶液中的自由能相关。有趣的是,烷基化会显着影响磷酸组氨酸衍生物在非常酸性的 pH 值下的稳定性。这些结果提供了对组氨酸磷酸化的关键机制见解,并将有助于设计未来的探针来研究酶促组氨酸磷酸化。
更新日期:2020-02-11
中文翻译:

生物正交 ATP 类似物用于评估组氨酸激酶自磷酸化的机制研究。
磷酸化是一种必不可少的蛋白质修饰,最常见的是通过三磷酸腺苷 (ATP) 底物与含羟基的氨基酸相关联。在过去的几十年里,人们对无数氨基酸的磷酸化在生物信号、代谢和基因转录中所起的作用有了更大的认识。组氨酸磷酸化发生在真核生物和原核生物中,但由于其在微生物双组分系统中的作用,已被证明在后者中占主导地位。由于其固有的不稳定性以及这种蛋白质修饰很少见的历史观点,研究组氨酸磷酸化的方法落后于研究丝氨酸、苏氨酸和酪氨酸修饰的方法。克服磷酸组氨酸反应性的一个重要策略是开发具有改变化学性质的基于底物的探针,以提高修饰寿命,但不会受到蛋白质识别或转移的影响。在这里,我们结合实验和计算研究,通过比较天然激酶底物、ATP 和烷基化 ATP 衍生物,更好地了解有效组氨酸磷酸化的分子要求。虽然组氨酸激酶对底物的识别是形成磷酸组氨酸衍生物的重要参数,但反应空间也影响结果。此外,我们发现所得磷酸组氨酸部分的稳定性与其水解产物的稳定性相关,特别是与它们在溶液中的自由能相关。有趣的是,烷基化会显着影响磷酸组氨酸衍生物在非常酸性的 pH 值下的稳定性。这些结果提供了对组氨酸磷酸化的关键机制见解,并将有助于设计未来的探针来研究酶促组氨酸磷酸化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号