Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cryo-EM structures of PAC1 receptor reveal ligand binding mechanism.
Cell Research ( IF 44.1 ) Pub Date : 2020-02-11 , DOI: 10.1038/s41422-020-0280-2 Jia Wang 1 , Xianqiang Song 2 , Dandan Zhang 2 , Xiaoqing Chen 2 , Xun Li 2 , Yaping Sun 2 , Cui Li 2 , Yunpeng Song 2 , Yao Ding 2 , Ruobing Ren 3 , Essa Hu Harrington 4 , Liaoyuan A Hu 2 , Wenge Zhong 2 , Cen Xu 5 , Xin Huang 6 , Hong-Wei Wang 1 , Yingli Ma 2
Cell Research ( IF 44.1 ) Pub Date : 2020-02-11 , DOI: 10.1038/s41422-020-0280-2 Jia Wang 1 , Xianqiang Song 2 , Dandan Zhang 2 , Xiaoqing Chen 2 , Xun Li 2 , Yaping Sun 2 , Cui Li 2 , Yunpeng Song 2 , Yao Ding 2 , Ruobing Ren 3 , Essa Hu Harrington 4 , Liaoyuan A Hu 2 , Wenge Zhong 2 , Cen Xu 5 , Xin Huang 6 , Hong-Wei Wang 1 , Yingli Ma 2
Affiliation
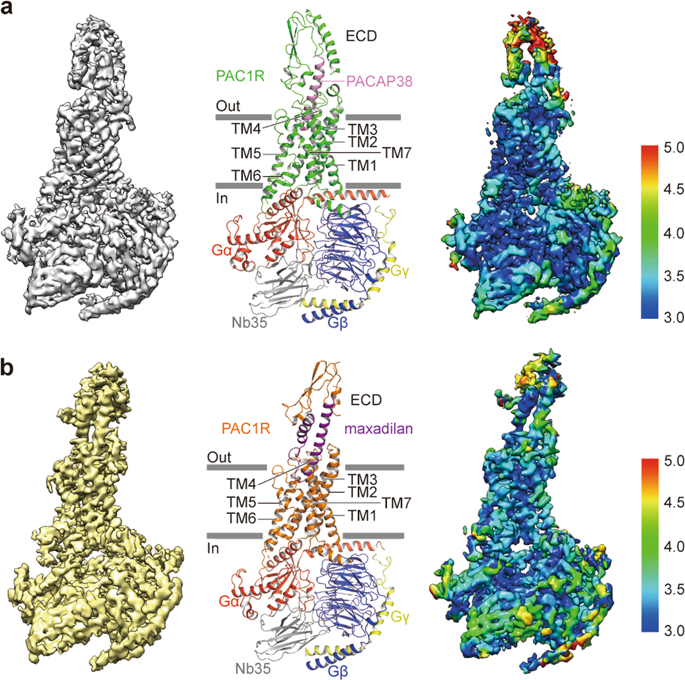
|
The pituitary adenylate cyclase-activating polypeptide type I receptor (PAC1R) belongs to the secretin receptor family and is widely distributed in the central neural system and peripheral organs. Abnormal activation of the receptor mediates trigeminovascular activation and sensitization, which is highly related to migraine, making PAC1R a potential therapeutic target. Elucidation of PAC1R activation mechanism would benefit discovery of therapeutic drugs for neuronal disorders. PAC1R activity is governed by pituitary adenylate cyclase-activating polypeptide (PACAP), known as a major vasodilator neuropeptide, and maxadilan, a native peptide from the sand fly, which is also capable of activating the receptor with similar potency. These peptide ligands have divergent sequences yet initiate convergent PAC1R activity. It is of interest to understand the mechanism of PAC1R ligand recognition and receptor activity regulation through structural biology. Here we report two near-atomic resolution cryo-EM structures of PAC1R activated by PACAP38 or maxadilan, providing structural insights into two distinct ligand binding modes. The structures illustrate flexibility of the extracellular domain (ECD) for ligands with distinct conformations, where ECD accommodates ligands in different orientations while extracellular loop 1 (ECL1) protrudes to further anchor the ligand bound in the orthosteric site. By structure-guided molecular modeling and mutagenesis, we tested residues in the ligand-binding pockets and identified clusters of residues that are critical for receptor activity. The structures reported here for the first time elucidate the mechanism of specificity and flexibility of ligand recognition and binding for PAC1R, and provide insights toward the design of therapeutic molecules targeting PAC1R.
中文翻译:

PAC1受体的Cryo-EM结构揭示了配体结合机制。
垂体腺苷酸环化酶激活多肽I型受体(PAC1R)属于secretin受体家族,广泛分布于中枢神经系统和周围器官。受体的异常激活介导与偏头痛高度相关的三叉神经血管激活和敏化,使PAC1R成为潜在的治疗靶标。PAC1R激活机制的阐明将有利于发现神经元疾病的治疗药物。PAC1R活性受垂体腺苷酸环化酶激活多肽(PACAP)(称为主要血管舒张神经肽)和maxadilan(一种来自沙蝇的天然肽)的控制,后者也能够以相似的效能激活该受体。这些肽配体具有不同的序列,但启动了聚合的PAC1R活性。通过结构生物学了解PAC1R配体识别和受体活性调节的机制是令人感兴趣的。在这里,我们报告由PACAP38或maxadilan激活的PAC1R的两个近原子分辨率的cryo-EM结构,为两种不同的配体结合模式提供结构见解。该结构说明了具有不同构象的配体的细胞外结构域(ECD)的柔性,其中ECD以不同的方向容纳配体,而细胞外环1(ECL1)突出以进一步锚定结合在正构位点上的配体。通过结构指导的分子建模和诱变,我们测试了配体结合口袋中的残基,并鉴定了对受体活性至关重要的残基簇。
更新日期:2020-02-11
中文翻译:

PAC1受体的Cryo-EM结构揭示了配体结合机制。
垂体腺苷酸环化酶激活多肽I型受体(PAC1R)属于secretin受体家族,广泛分布于中枢神经系统和周围器官。受体的异常激活介导与偏头痛高度相关的三叉神经血管激活和敏化,使PAC1R成为潜在的治疗靶标。PAC1R激活机制的阐明将有利于发现神经元疾病的治疗药物。PAC1R活性受垂体腺苷酸环化酶激活多肽(PACAP)(称为主要血管舒张神经肽)和maxadilan(一种来自沙蝇的天然肽)的控制,后者也能够以相似的效能激活该受体。这些肽配体具有不同的序列,但启动了聚合的PAC1R活性。通过结构生物学了解PAC1R配体识别和受体活性调节的机制是令人感兴趣的。在这里,我们报告由PACAP38或maxadilan激活的PAC1R的两个近原子分辨率的cryo-EM结构,为两种不同的配体结合模式提供结构见解。该结构说明了具有不同构象的配体的细胞外结构域(ECD)的柔性,其中ECD以不同的方向容纳配体,而细胞外环1(ECL1)突出以进一步锚定结合在正构位点上的配体。通过结构指导的分子建模和诱变,我们测试了配体结合口袋中的残基,并鉴定了对受体活性至关重要的残基簇。



























 京公网安备 11010802027423号
京公网安备 11010802027423号