Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Allosteric Activation of PI3Kα Results in Dynamic Access to Catalytically Competent Conformations.
Structure ( IF 5.7 ) Pub Date : 2020-02-10 , DOI: 10.1016/j.str.2020.01.010 Mayukh Chakrabarti 1 , Sandra B Gabelli 2 , L Mario Amzel 1
Structure ( IF 5.7 ) Pub Date : 2020-02-10 , DOI: 10.1016/j.str.2020.01.010 Mayukh Chakrabarti 1 , Sandra B Gabelli 2 , L Mario Amzel 1
Affiliation
|
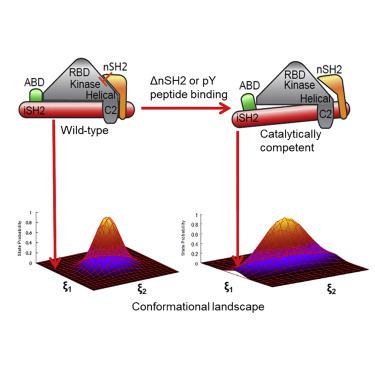
Class I phosphoinositide-3-kinases (PI3Ks) phosphorylate PIP2 at its 3' inositol position to generate PIP3, a second messenger that influences signaling cascades regulating cellular growth, survival, and proliferation. Previous studies have suggested that PI3Kα activation involves dislodging the p85α nSH2 domain from the p110α catalytic subunit by binding activated receptor tyrosine kinases. We carried out molecular dynamics simulations to determine, mechanistically and structurally, how PI3Kα conformations are influenced by physiological effectors and the nSH2 domain. We demonstrate that changes in protein dynamics mediated by allosteric regulation significantly increase the population of catalytically competent states without changing the enzyme ground-state structure. Furthermore, we demonstrate that modulation of active-site residue interactions with enzyme substrates can reciprocally influence nSH2 domain dynamics. Together, these results suggest that dynamic allostery plays a role in populating the catalytically competent conformation of PI3Kα, and provide a key platform for the design of novel chemotherapeutic PI3Kα inhibitors.
中文翻译:

PI3Kα的变构活化可动态获得催化活性构象。
I类磷酸肌醇-3-激酶(PI3K)在其3'肌醇位置使PIP2磷酸化,生成PIP3,PIP3是第二个信使,影响信号级联调节细胞的生长,存活和增殖。先前的研究表明,PI3Kα激活涉及通过结合激活的受体酪氨酸激酶从p110α催化亚基上去除p85αnSH2结构域。我们进行了分子动力学模拟,从机理和结构上确定了PI3Kα构象如何受到生理效应子和nSH2结构域的影响。我们证明变构调节介导的蛋白质动力学变化显着增加了催化感受态的数量,而没有改变酶基态的结构。此外,我们证明,活性位点残基与酶底物相互作用的调节可以相互影响nSH2域动力学。总之,这些结果表明动态变构作用在填充PI3Kα的催化有效构象中起作用,并为设计新型化疗PI3Kα抑制剂提供了关键平台。
更新日期:2020-02-10
中文翻译:

PI3Kα的变构活化可动态获得催化活性构象。
I类磷酸肌醇-3-激酶(PI3K)在其3'肌醇位置使PIP2磷酸化,生成PIP3,PIP3是第二个信使,影响信号级联调节细胞的生长,存活和增殖。先前的研究表明,PI3Kα激活涉及通过结合激活的受体酪氨酸激酶从p110α催化亚基上去除p85αnSH2结构域。我们进行了分子动力学模拟,从机理和结构上确定了PI3Kα构象如何受到生理效应子和nSH2结构域的影响。我们证明变构调节介导的蛋白质动力学变化显着增加了催化感受态的数量,而没有改变酶基态的结构。此外,我们证明,活性位点残基与酶底物相互作用的调节可以相互影响nSH2域动力学。总之,这些结果表明动态变构作用在填充PI3Kα的催化有效构象中起作用,并为设计新型化疗PI3Kα抑制剂提供了关键平台。


























 京公网安备 11010802027423号
京公网安备 11010802027423号