Journal of Organometallic Chemistry ( IF 2.3 ) Pub Date : 2020-02-10 , DOI: 10.1016/j.jorganchem.2020.121174 Vladimir N. Mikhaylov , Viktor N. Sorokoumov , Alexander S. Novikov , Maria V. Melnik , Alexander G. Tskhovrebov , Irina A. Balova
|
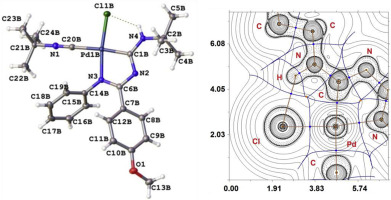
Aromatic amidines 1a-e undergo facile reaction with one isocyanide in PdCl2(CNBut)2 giving carbene complexes 2b-d (Scheme 2) in high isolated yields (79–95%). The structures of 2b–d were confirmed by the 1H and 13C NMR spectroscopies, high resolution electrospray ionization mass spectrometry (HRESI-MS), IR, the elemental analyses (C, H, N), and X-ray diffraction analysis for 2c, which revealed that the carbene and unreacted isocyanide ligands were located in a mutually trans position. Such arrangement was unexpected since it did not fit trans effect rule. Stabilization of the unfavorable isomer was rationalized by intramolecular hydrogen bonding. The nature of the intramolecular non-covalent interactions, which were responsible for the stabilization of the trans-isomer, was studied theoretically using DFT calculations and topological analysis of the electron density distribution within the framework of Bader's theory (QTAIM method).
中文翻译:

分子内氢键稳定了卡宾/异氰化物混合钯II配合物中的反式构型
芳香am 1a-e与一种异氰酸酯在PdCl 2(CNBu t)2中容易反应,从而以高分离产率(79-95%)得到卡宾络合物2b-d(方案2)。通过1 H和13 C NMR光谱,高分辨率电喷雾电离质谱(HRESI-MS),IR,元素分析(C,H,N)和X射线衍射分析确定了2b–d的结构如图2c所示,其揭示了卡宾和未反应的异氰酸酯配体位于相互反位。这样的安排是出乎意料的,因为它不适合跨效果规则。不利的异构体的稳定通过分子内氢键来合理化。分子内非共价相互作用的本质,负责反式异构体的稳定,是在巴德尔理论(QTAIM方法)的框架内使用DFT计算和电子密度分布的拓扑分析对理论进行了研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号