当前位置:
X-MOL 学术
›
Cell Death Differ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Drp1 overexpression induces desmin disassembling and drives kinesin-1 activation promoting mitochondrial trafficking in skeletal muscle.
Cell Death and Differentiation ( IF 12.4 ) Pub Date : 2020-02-10 , DOI: 10.1038/s41418-020-0510-7 Matteo Giovarelli 1 , Silvia Zecchini 1 , Emanuele Martini 2 , Massimiliano Garrè 2 , Sara Barozzi 2 , Michela Ripolone 3 , Laura Napoli 3 , Marco Coazzoli 1 , Chiara Vantaggiato 4 , Paulina Roux-Biejat 1 , Davide Cervia 5 , Claudia Moscheni 1 , Cristiana Perrotta 1 , Dario Parazzoli 2 , Emilio Clementi 1, 4 , Clara De Palma 6
Cell Death and Differentiation ( IF 12.4 ) Pub Date : 2020-02-10 , DOI: 10.1038/s41418-020-0510-7 Matteo Giovarelli 1 , Silvia Zecchini 1 , Emanuele Martini 2 , Massimiliano Garrè 2 , Sara Barozzi 2 , Michela Ripolone 3 , Laura Napoli 3 , Marco Coazzoli 1 , Chiara Vantaggiato 4 , Paulina Roux-Biejat 1 , Davide Cervia 5 , Claudia Moscheni 1 , Cristiana Perrotta 1 , Dario Parazzoli 2 , Emilio Clementi 1, 4 , Clara De Palma 6
Affiliation
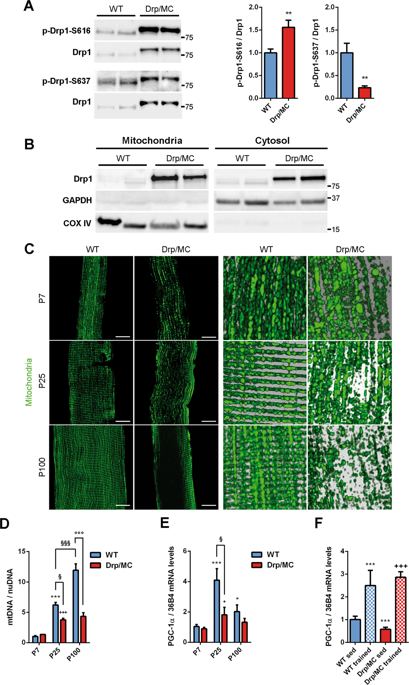
|
Mitochondria change distribution across cells following a variety of pathophysiological stimuli. The mechanisms presiding over this redistribution are yet undefined. In a murine model overexpressing Drp1 specifically in skeletal muscle, we find marked mitochondria repositioning in muscle fibres and we demonstrate that Drp1 is involved in this process. Drp1 binds KLC1 and enhances microtubule-dependent transport of mitochondria. Drp1-KLC1 coupling triggers the displacement of KIF5B from kinesin-1 complex increasing its binding to microtubule tracks and mitochondrial transport. High levels of Drp1 exacerbate this mechanism leading to the repositioning of mitochondria closer to nuclei. The reduction of Drp1 levels decreases kinesin-1 activation and induces the partial recovery of mitochondrial distribution. Drp1 overexpression is also associated with higher cyclin-dependent kinase-1 (Cdk-1) activation that promotes the persistent phosphorylation of desmin at Ser-31 and its disassembling. Fission inhibition has a positive effect on desmin Ser-31 phosphorylation, regardless of Cdk-1 activation, suggesting that induction of both fission and Cdk-1 are required for desmin collapse. This altered desmin architecture impairs mechanotransduction and compromises mitochondrial network stability priming mitochondria transport through microtubule-dependent trafficking with a mechanism that involves the Drp1-dependent regulation of kinesin-1 complex.
中文翻译:

Drp1 过表达诱导结蛋白分解并驱动驱动蛋白-1 激活,促进骨骼肌中的线粒体运输。
在各种病理生理刺激后,线粒体改变细胞间的分布。主持这种重新分配的机制尚未确定。在一个专门在骨骼肌中过度表达 Drp1 的小鼠模型中,我们发现肌肉纤维中有明显的线粒体重新定位,我们证明 Drp1 参与了这个过程。Drp1 结合 KLC1 并增强线粒体的微管依赖性转运。Drp1-KLC1 偶联触发 KIF5B 从驱动蛋白-1 复合物的置换,增加其与微管轨迹和线粒体转运的结合。高水平的 Drp1 加剧了这种机制,导致线粒体重新定位更靠近细胞核。Drp1 水平的降低会降低驱动蛋白-1 的激活并诱导线粒体分布的部分恢复。Drp1 过度表达还与更高的细胞周期蛋白依赖性激酶 1 (Cdk-1) 激活相关,后者促进了 Ser-31 处结蛋白的持续磷酸化及其分解。裂变抑制对结蛋白 Ser-31 磷酸化有积极影响,无论 Cdk-1 激活如何,表明裂变和 Cdk-1 的诱导都是结蛋白崩溃所必需的。这种改变的结蛋白结构损害了机械转导并损害了线粒体网络稳定性,通过微管依赖性运输启动线粒体运输,其机制涉及驱动蛋白 1 复合物的 Drp1 依赖性调节。无论 Cdk-1 激活如何,表明裂变和 Cdk-1 的诱导都是结蛋白崩溃所必需的。这种改变的结蛋白结构损害了机械转导并损害了线粒体网络稳定性,通过微管依赖性运输启动线粒体运输,其机制涉及驱动蛋白 1 复合物的 Drp1 依赖性调节。无论 Cdk-1 激活如何,表明裂变和 Cdk-1 的诱导都是结蛋白崩溃所必需的。这种改变的结蛋白结构损害了机械转导并损害了线粒体网络稳定性,通过微管依赖性运输启动线粒体运输,其机制涉及驱动蛋白 1 复合物的 Drp1 依赖性调节。
更新日期:2020-02-10
中文翻译:

Drp1 过表达诱导结蛋白分解并驱动驱动蛋白-1 激活,促进骨骼肌中的线粒体运输。
在各种病理生理刺激后,线粒体改变细胞间的分布。主持这种重新分配的机制尚未确定。在一个专门在骨骼肌中过度表达 Drp1 的小鼠模型中,我们发现肌肉纤维中有明显的线粒体重新定位,我们证明 Drp1 参与了这个过程。Drp1 结合 KLC1 并增强线粒体的微管依赖性转运。Drp1-KLC1 偶联触发 KIF5B 从驱动蛋白-1 复合物的置换,增加其与微管轨迹和线粒体转运的结合。高水平的 Drp1 加剧了这种机制,导致线粒体重新定位更靠近细胞核。Drp1 水平的降低会降低驱动蛋白-1 的激活并诱导线粒体分布的部分恢复。Drp1 过度表达还与更高的细胞周期蛋白依赖性激酶 1 (Cdk-1) 激活相关,后者促进了 Ser-31 处结蛋白的持续磷酸化及其分解。裂变抑制对结蛋白 Ser-31 磷酸化有积极影响,无论 Cdk-1 激活如何,表明裂变和 Cdk-1 的诱导都是结蛋白崩溃所必需的。这种改变的结蛋白结构损害了机械转导并损害了线粒体网络稳定性,通过微管依赖性运输启动线粒体运输,其机制涉及驱动蛋白 1 复合物的 Drp1 依赖性调节。无论 Cdk-1 激活如何,表明裂变和 Cdk-1 的诱导都是结蛋白崩溃所必需的。这种改变的结蛋白结构损害了机械转导并损害了线粒体网络稳定性,通过微管依赖性运输启动线粒体运输,其机制涉及驱动蛋白 1 复合物的 Drp1 依赖性调节。无论 Cdk-1 激活如何,表明裂变和 Cdk-1 的诱导都是结蛋白崩溃所必需的。这种改变的结蛋白结构损害了机械转导并损害了线粒体网络稳定性,通过微管依赖性运输启动线粒体运输,其机制涉及驱动蛋白 1 复合物的 Drp1 依赖性调节。


























 京公网安备 11010802027423号
京公网安备 11010802027423号