Nature Catalysis ( IF 37.8 ) Pub Date : 2020-02-10 , DOI: 10.1038/s41929-019-0420-6 Zhi Zhou , Gerard Roelfes
|
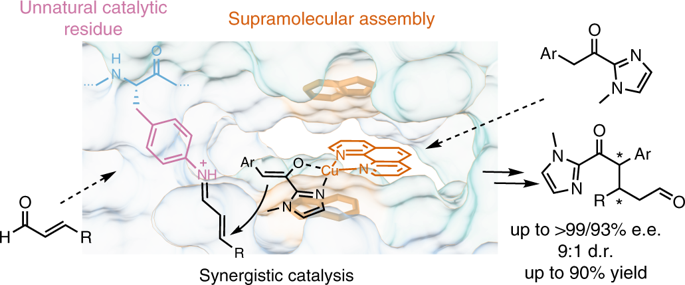
Artificial enzymes, which are hybrids of proteins with abiological catalytic groups, have emerged as a powerful approach towards the creation of enzymes for new-to-nature reactions. Typically, only a single abiological catalytic moiety is incorporated. Here we introduce a design of an artificial enzyme that comprises two different abiological catalytic moieties and show that these can act synergistically to achieve high activity and enantioselectivity (up to >99% e.e.) in the catalysed Michael addition reaction. The design is based on the lactococcal multidrug resistance regulator as the protein scaffold and combines a genetically encoded unnatural p-aminophenylalanine residue (which activates an enal through iminium ion formation) and a supramolecularly bound Lewis acidic Cu(ii) complex (which activates the Michael donor by enolization and delivers it to one preferred prochiral face of the activated enal). This study demonstrates that synergistic combination of abiological catalytic groups is a robust way to achieve catalysis that is normally outside of the realm of artificial enzymes.
中文翻译:

通过两个非生物催化位点的同时作用,在人工酶中进行协同催化
人工酶是具有非生物催化基团的蛋白质的杂合体,已成为创建用于新自然反应的酶的有力方法。通常,仅掺入单个非生物催化部分。在这里,我们介绍了一种人造酶的设计,该酶包含两个不同的非生物催化部分,并表明它们可以协同作用,以在催化的迈克尔加成反应中实现高活性和对映选择性(最高> 99%ee)。该设计基于作为蛋白质支架的乳球菌多药耐药性调节剂,并结合了遗传编码的非天然对氨基苯丙氨酸残基(可通过亚胺离子的形成来激活烯)和超分子结合的路易斯酸性Cu(ii)。)配合物(通过烯醇化作用激活迈克尔供体并将其传递到活化的烯类的一个较好的手性前脸)。这项研究表明,生物催化基团的协同结合是实现催化的一种可靠方法,而催化通常是在人造酶领域之外的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号