当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Activity adaptability of a DhHP-6 peroxidase-mimic in wide pH and temperature ranges and solvent media
Catalysis Science & Technology ( IF 5 ) Pub Date : 2020/02/06 , DOI: 10.1039/c9cy01855g Jiaqing Yan 1, 2, 3, 4, 5 , Zhengqiang Li 1, 2, 3, 4, 5 , Min Liu 3, 5, 6, 7 , Xiaoli Sun 3, 5, 8, 9 , Li Ma 10, 11, 12, 13 , Zhi Wang 1, 2, 3, 4, 5 , Zijian Zhao 5, 7, 14, 15 , Xuri Huang 3, 5, 8, 9 , Long Yuan 5, 16, 17, 18, 19
Catalysis Science & Technology ( IF 5 ) Pub Date : 2020/02/06 , DOI: 10.1039/c9cy01855g Jiaqing Yan 1, 2, 3, 4, 5 , Zhengqiang Li 1, 2, 3, 4, 5 , Min Liu 3, 5, 6, 7 , Xiaoli Sun 3, 5, 8, 9 , Li Ma 10, 11, 12, 13 , Zhi Wang 1, 2, 3, 4, 5 , Zijian Zhao 5, 7, 14, 15 , Xuri Huang 3, 5, 8, 9 , Long Yuan 5, 16, 17, 18, 19
Affiliation
|
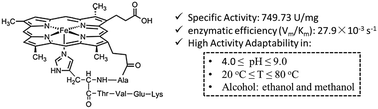
Separating the active units while maintaining the majority of the functions of enzymes seems to be a paradox and therefore challenging, not to mention also improving the adaptability to harsh conditions simultaneously. In this paper, we demonstrate that a well-designed hexapeptide molecule, deuterohemin-β-Ala-His-Thr-Val-Glu-Lys (DhHP-6), adapted from cytochrome c, shows superior peroxidase activity over a wide pH and temperature range. The activity of DhHP-6 was first assessed via three substrates (i.e., 2,2′azinodi(3-ethylbenzthiazoline)-6-sulfonate (ABTS), 1,2,3-trihydroxybenzene (THB) and phenol) with reference to the classically studied examples microperoxidase (MP-11) and horseradish peroxidase (HRP). The environmental adaptability was assessed via three factors: pH, temperature, and the polarity of solvents. In the phenol substrate model reaction, DhHP-6 shows superior specific activity (749.73 U mg−1) and adaptability compared to HRP (311.32 U mg−1). Under the optimum solvent conditions (15% methanol by volume ratio), the enzymatic efficiency (Vm/Km) of DhHP-6 reached 27.9 × 10−3 s−1, 16-times higher than that of HRP (1.81 × 10−3 s−1). The methanol concentration dependent electron paramagnetic spectra show an axial-promoted intermediate process for the phenol catalytic reaction. This work provides a protocol and feasible methodology for the design and fabrication of natural enzyme mimicking small molecules that maintain most of their native activity for better biocatalytic applications in organic pollutant remediation and other related processes.
中文翻译:

DhHP-6过氧化物酶模拟物在宽pH和温度范围以及溶剂介质中的活性适应性
在保持酶的大部分功能的同时分离活性单位似乎是一个悖论,因此具有挑战性,更不用说同时提高对恶劣条件的适应性。在本文中,我们证明了一种经过精心设计的六肽分子,氘铁血红素-β-Ala-His-Thr-Val-Glu-Lys(DhHP-6),适用于细胞色素c,在较宽的pH和温度下均表现出优异的过氧化物酶活性范围。DhHP-6的活性,首先评估通过三个基板(即,2,2'azinodi(3-乙基)-6-磺酸酯(ABTS),1,2,3-三羟基苯(THB)和苯酚),参照经典研究的例子有微过氧化物酶(MP-11)和辣根过氧化物酶(HRP)。评估环境适应性通过三个因素:pH,温度和溶剂的极性。在酚底物模型反应中,与HRP(311.32 U mg -1)相比,DhHP-6具有优越的比活(749.73 U mg -1)和适应性。在最佳溶剂条件下(体积比为15%的甲醇),DhHP-6的酶效率(V m / K m)达到27.9×10 -3 s -1,比HRP(1.81×10)高16倍。−3 s −1)。甲醇浓度相关的电子顺磁光谱显示了苯酚催化反应的轴向促进中间过程。这项工作为模仿和模仿小分子的天然酶的设计和制造提供了一种协议和可行的方法,这些分子保留了其大部分天然活性,可以在有机污染物修复和其他相关过程中更好地进行生物催化。
更新日期:2020-03-26
中文翻译:

DhHP-6过氧化物酶模拟物在宽pH和温度范围以及溶剂介质中的活性适应性
在保持酶的大部分功能的同时分离活性单位似乎是一个悖论,因此具有挑战性,更不用说同时提高对恶劣条件的适应性。在本文中,我们证明了一种经过精心设计的六肽分子,氘铁血红素-β-Ala-His-Thr-Val-Glu-Lys(DhHP-6),适用于细胞色素c,在较宽的pH和温度下均表现出优异的过氧化物酶活性范围。DhHP-6的活性,首先评估通过三个基板(即,2,2'azinodi(3-乙基)-6-磺酸酯(ABTS),1,2,3-三羟基苯(THB)和苯酚),参照经典研究的例子有微过氧化物酶(MP-11)和辣根过氧化物酶(HRP)。评估环境适应性通过三个因素:pH,温度和溶剂的极性。在酚底物模型反应中,与HRP(311.32 U mg -1)相比,DhHP-6具有优越的比活(749.73 U mg -1)和适应性。在最佳溶剂条件下(体积比为15%的甲醇),DhHP-6的酶效率(V m / K m)达到27.9×10 -3 s -1,比HRP(1.81×10)高16倍。−3 s −1)。甲醇浓度相关的电子顺磁光谱显示了苯酚催化反应的轴向促进中间过程。这项工作为模仿和模仿小分子的天然酶的设计和制造提供了一种协议和可行的方法,这些分子保留了其大部分天然活性,可以在有机污染物修复和其他相关过程中更好地进行生物催化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号