当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Changes in mammalian copper homeostasis during microbial infection.
Metallomics ( IF 3.4 ) Pub Date : 2020-01-24 , DOI: 10.1039/c9mt00294d Edward M Culbertson 1 , Aslam A Khan 2 , Abigael Muchenditsi 3 , Svetlana Lutsenko 3 , David J Sullivan 4 , Michael J Petris 2 , Brendan P Cormack 5 , Valeria C Culotta 1
Metallomics ( IF 3.4 ) Pub Date : 2020-01-24 , DOI: 10.1039/c9mt00294d Edward M Culbertson 1 , Aslam A Khan 2 , Abigael Muchenditsi 3 , Svetlana Lutsenko 3 , David J Sullivan 4 , Michael J Petris 2 , Brendan P Cormack 5 , Valeria C Culotta 1
Affiliation
|
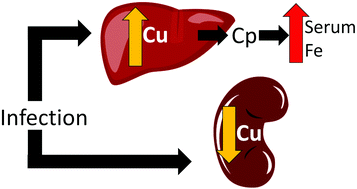
Animals carefully control homeostasis of Cu, a metal that is both potentially toxic and an essential nutrient. During infection, various shifts in Cu homeostasis can ensue. In mice infected with Candida albicans, serum Cu progressively rises and at late stages of infection, liver Cu rises, while kidney Cu declines. The basis for these changes in Cu homeostasis was poorly understood. We report here that the progressive rise in serum Cu is attributable to liver production of the multicopper oxidase ceruloplasmin (Cp). Through studies using Cp-/- mice, we find this elevated Cp helps recover serum Fe levels at late stages of infection, consistent with a role for Cp in loading transferrin with Fe. Cp also accounts for the elevation in liver Cu seen during infection, but not for the fluctuations in kidney Cu. The Cu exporting ATPase ATP7B is one candidate for kidney Cu control, but we find no change in the pattern of kidney Cu loss during infection of Atp7b-/- mice, implying alternative mechanisms. To test whether fungal infiltration of kidney tissue was required for kidney Cu loss, we explored other paradigms of infection. Infection with the intravascular malaria parasite Plasmodium berghei caused a rise in serum Cu and decrease in kidney Cu similar to that seen with C. albicans. Thus, dynamics in kidney Cu homeostasis appear to be a common feature among vastly different infection paradigms. The implications for such Cu homeostasis control in immunity are discussed.
中文翻译:

在微生物感染过程中哺乳动物铜稳态的变化。
动物小心地控制了铜的稳态,铜是一种潜在的有毒物质,也是一种必需的营养物质。在感染过程中,可能会发生铜稳态的各种变化。在感染白色念珠菌的小鼠中,血清Cu逐渐升高,在感染后期,肝脏Cu升高,而肾脏Cu降低。铜稳态的这些变化的基础了解甚少。我们在这里报告,血清铜的逐步升高归因于多铜氧化酶铜蓝蛋白(Cp)的肝脏生产。通过使用Cp-/-小鼠进行的研究,我们发现这种升高的Cp有助于在感染后期恢复血清中的Fe水平,这与Cp在将转铁蛋白负载Fe中的作用一致。Cp还可以解释感染期间肝脏Cu的升高,但不能解释肾脏Cu的波动。铜输出ATPase ATP7B是控制肾脏铜的一种候选药物,但是我们发现在Atp7b-/-小鼠感染期间肾脏铜丢失的模式没有变化,这暗示了其他机制。为了测试是否需要肾脏组织的真菌浸润以减少肾脏铜的流失,我们探索了其他感染范例。血管内疟原虫感染伯氏疟原虫引起血清铜的升高和肾脏铜的降低,与白色念珠菌相似。因此,肾脏铜稳态的动态变化似乎是极为不同的感染范例之间的共同特征。讨论了这种铜稳态控制免疫力的意义。为了测试是否需要肾脏组织的真菌浸润以减少肾脏铜的流失,我们探索了其他感染范例。血管内疟原虫感染伯氏疟原虫导致血清铜的升高和肾脏铜的降低,与白色念珠菌相似。因此,肾脏铜稳态的动态变化似乎是极为不同的感染范例之间的共同特征。讨论了这种铜稳态控制免疫力的意义。为了测试是否需要肾脏组织的真菌浸润以减少肾脏铜的流失,我们探索了其他感染范例。血管内疟原虫感染伯氏疟原虫导致血清铜的升高和肾脏铜的降低,与白色念珠菌相似。因此,肾脏铜稳态的动态变化似乎是极为不同的感染范例之间的共同特征。讨论了这种铜稳态控制免疫力的意义。
更新日期:2020-03-26
中文翻译:

在微生物感染过程中哺乳动物铜稳态的变化。
动物小心地控制了铜的稳态,铜是一种潜在的有毒物质,也是一种必需的营养物质。在感染过程中,可能会发生铜稳态的各种变化。在感染白色念珠菌的小鼠中,血清Cu逐渐升高,在感染后期,肝脏Cu升高,而肾脏Cu降低。铜稳态的这些变化的基础了解甚少。我们在这里报告,血清铜的逐步升高归因于多铜氧化酶铜蓝蛋白(Cp)的肝脏生产。通过使用Cp-/-小鼠进行的研究,我们发现这种升高的Cp有助于在感染后期恢复血清中的Fe水平,这与Cp在将转铁蛋白负载Fe中的作用一致。Cp还可以解释感染期间肝脏Cu的升高,但不能解释肾脏Cu的波动。铜输出ATPase ATP7B是控制肾脏铜的一种候选药物,但是我们发现在Atp7b-/-小鼠感染期间肾脏铜丢失的模式没有变化,这暗示了其他机制。为了测试是否需要肾脏组织的真菌浸润以减少肾脏铜的流失,我们探索了其他感染范例。血管内疟原虫感染伯氏疟原虫引起血清铜的升高和肾脏铜的降低,与白色念珠菌相似。因此,肾脏铜稳态的动态变化似乎是极为不同的感染范例之间的共同特征。讨论了这种铜稳态控制免疫力的意义。为了测试是否需要肾脏组织的真菌浸润以减少肾脏铜的流失,我们探索了其他感染范例。血管内疟原虫感染伯氏疟原虫导致血清铜的升高和肾脏铜的降低,与白色念珠菌相似。因此,肾脏铜稳态的动态变化似乎是极为不同的感染范例之间的共同特征。讨论了这种铜稳态控制免疫力的意义。为了测试是否需要肾脏组织的真菌浸润以减少肾脏铜的流失,我们探索了其他感染范例。血管内疟原虫感染伯氏疟原虫导致血清铜的升高和肾脏铜的降低,与白色念珠菌相似。因此,肾脏铜稳态的动态变化似乎是极为不同的感染范例之间的共同特征。讨论了这种铜稳态控制免疫力的意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号