当前位置:
X-MOL 学术
›
Environ. Sci.: Processes Impacts
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Isotope fractionation (2H/1H, 13C/12C, 37Cl/35Cl) in trichloromethane and trichloroethene caused by partitioning between gas phase and water.
Environmental Science: Processes & Impacts ( IF 5.5 ) Pub Date : 2020-01-23 , DOI: 10.1039/c9em00583h Axel Horst 1 , Georges Lacrampe-Couloume
Environmental Science: Processes & Impacts ( IF 5.5 ) Pub Date : 2020-01-23 , DOI: 10.1039/c9em00583h Axel Horst 1 , Georges Lacrampe-Couloume
Affiliation
|
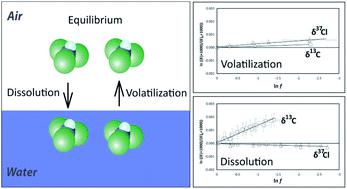
Transfer of organic compounds between aqueous and gaseous phases may change the isotopic composition which complicates the isotopic characterization of sources and transformation mechanisms in environmental samples. Studies investigating kinetic phase transfer of compounds dissolved in water (volatilization) are scarce, even though it presents an environmentally very relevant phase transfer scenario. In the current study, the occurrence of kinetic isotope fractionation (2H/1H, 13C/12C, 37Cl/35Cl) was investigated for two volatile organic compounds (trichloroethene, TCE and trichloromethane, TCM) during volatilization from water and gas-phase dissolution in water. In addition, experiments were also carried out at equilibrium conditions. The results indicated that volatilization of trichloromethane and trichloroethene from water, in contrast to pure phase evaporation, only caused small (chlorine) or negligible (hydrogen, carbon) isotope fractionation whereas for dissolution in water significant carbon isotope effects were found. At equilibrium conditions, hydrogen and carbon isotopes showed significant differences between dissolved and gaseous phase whereas small to insignificant differences were measured for chlorine isotopes. The results confirm the hypothesis that isotope effects during volatilization of organics from water are caused by transport inhibition in the aqueous phase. The consideration of gas-phase diffusion and vapor pressure isotope effects (Craig-Gordon model) could not reproduce the measured isotopic data. Overall, this study provides an overview of the most common kinetic and equilibrium partitioning scenarios and reports associated isotope effects. As such it illustrates under which environmental conditions isotopic signatures of chlorinated volatile organics may change, or remain constant, during transfer between surface waters and air.
中文翻译:

气相和水之间的分配引起的三氯甲烷和三氯乙烯中的同位素分馏(2H / 1H,13C / 12C,37Cl / 35Cl)。
有机化合物在水相和气相之间的转移可能会改变同位素组成,这会使环境样品中源的同位素表征和转化机制复杂化。很少有研究研究溶解在水中的化合物的动力学相转移(挥发)的方法,尽管它提出了与环境非常相关的相转移方案。在当前的研究中,研究了两种挥发性有机化合物(三氯乙烯,TCE和三氯甲烷,TCM)在从水中挥发和气相溶解过程中的动力学同位素分馏(2H / 1H,13C / 12C,37Cl / 35Cl)的发生。水。此外,实验也在平衡条件下进行。结果表明,三氯甲烷和三氯乙烯从水中挥发,与纯相蒸发相反,仅引起较小的(氯)或可忽略不计的(氢,碳)同位素分馏,而在水中溶解时,会发现明显的碳同位素效应。在平衡条件下,氢和碳同位素在溶解相和气相之间显示出显着差异,而氯同位素测得的差异很小至不明显。结果证实了这样的假设,即有机物从水中挥发过程中的同位素效应是由水相中的运输抑制引起的。气相扩散和蒸气压同位素效应(Craig-Gordon模型)的考虑无法重现测得的同位素数据。总体而言,本研究概述了最常见的动力学和平衡分配方案,并报告了相关的同位素效应。
更新日期:2020-01-23
中文翻译:

气相和水之间的分配引起的三氯甲烷和三氯乙烯中的同位素分馏(2H / 1H,13C / 12C,37Cl / 35Cl)。
有机化合物在水相和气相之间的转移可能会改变同位素组成,这会使环境样品中源的同位素表征和转化机制复杂化。很少有研究研究溶解在水中的化合物的动力学相转移(挥发)的方法,尽管它提出了与环境非常相关的相转移方案。在当前的研究中,研究了两种挥发性有机化合物(三氯乙烯,TCE和三氯甲烷,TCM)在从水中挥发和气相溶解过程中的动力学同位素分馏(2H / 1H,13C / 12C,37Cl / 35Cl)的发生。水。此外,实验也在平衡条件下进行。结果表明,三氯甲烷和三氯乙烯从水中挥发,与纯相蒸发相反,仅引起较小的(氯)或可忽略不计的(氢,碳)同位素分馏,而在水中溶解时,会发现明显的碳同位素效应。在平衡条件下,氢和碳同位素在溶解相和气相之间显示出显着差异,而氯同位素测得的差异很小至不明显。结果证实了这样的假设,即有机物从水中挥发过程中的同位素效应是由水相中的运输抑制引起的。气相扩散和蒸气压同位素效应(Craig-Gordon模型)的考虑无法重现测得的同位素数据。总体而言,本研究概述了最常见的动力学和平衡分配方案,并报告了相关的同位素效应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号