Chemical Physics ( IF 2.3 ) Pub Date : 2020-02-08 , DOI: 10.1016/j.chemphys.2020.110713 Gokhan Kacar
|
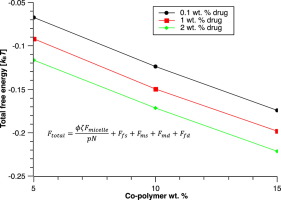
Thermodynamic stability of poloxamer micelles excluding and encapsulating ibuprofen drug at different concentrations is studied by means of free energies. Free energies are computed by a partitioning of the total free energy in order to consider the different parts of the co-polymers together with free co-polymer chains in solvent. Moreover, extra terms to the free energy are added to account for the drug-micelle and drug-solvent interactions. The thermodynamic stability of the system is found to be positively correlated with the drug loading for the same system. The RDFs computed between water and the drug molecules signify the decreasing neighboring between these pairs as the co-polymer and drug concentrations are increased in the mixtures as the system leans towards a higher thermodynamic stability. In this work, not only the thermodynamic stability but also its relation to the structure are discussed for a drug-laden system demonstrating the utility of the proposed procedure.
中文翻译:

布洛芬负载泊洛沙姆胶束的热力学稳定性
通过自由能研究了泊洛沙姆胶束的热力学稳定性,其中不包括和包封不同浓度的布洛芬药物。通过考虑总自由能的分配来计算自由能,以考虑共聚物的不同部分以及溶剂中的自由共聚物链。此外,添加了额外的自由能术语以解释药物-胶束和药物-溶剂的相互作用。发现该系统的热力学稳定性与相同系统的载药量正相关。在水和药物分子之间计算的RDF表示,随着体系向更高的热力学稳定性倾斜,随着混合物中共聚物和药物浓度的增加,这些对之间的邻域减少。在这项工作中


























 京公网安备 11010802027423号
京公网安备 11010802027423号