当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemically mediated nitrate reduction on nanoconfined zerovalent iron: Properties and mechanism.
Water Research ( IF 12.8 ) Pub Date : 2020-02-08 , DOI: 10.1016/j.watres.2020.115596 Zhenwei Liu 1 , Shangshang Dong 1 , Di Zou 2 , Jie Ding 1 , Anqing Yu 1 , Jing Zhang 1 , Chao Shan 3 , Guandao Gao 3 , Bingcai Pan 3
Water Research ( IF 12.8 ) Pub Date : 2020-02-08 , DOI: 10.1016/j.watres.2020.115596 Zhenwei Liu 1 , Shangshang Dong 1 , Di Zou 2 , Jie Ding 1 , Anqing Yu 1 , Jing Zhang 1 , Chao Shan 3 , Guandao Gao 3 , Bingcai Pan 3
Affiliation
|
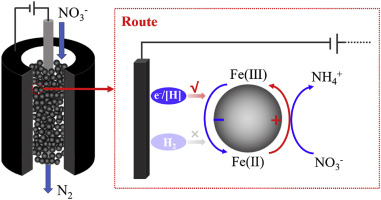
Selective reduction of nitrate to N2 is attractive but still a difficult challenge in the water treatment field. Herein, we established a flow-through electrochemical system packed with polymeric beads supported nZVI (nZVI@D201) for selective nitrate reduction. Consequently, efficient nitrate reduction in the flow mode was achieved on nZVI@D201 under electrochemical regulation with N2 selectivity of up to 95% for at least 60 h. Otherwise, nZVI was gradually exhausted after 20 h, and the product was mainly the undesired NH4+. Through a series of comparative experiments, we clarified that the enhanced nitrate reduction on nZVI under electrochemical regulation was mainly attributed to electrons (from cathode) and active hydrogen ([H]) rather than the previously speculated H2. Combining the characterizations of nZVI during nitrate reduction by X-ray diffraction and X-ray photoelectron spectrometry, we found that nitrate reduction under electrochemical regulation was mediated by nZVI along with the resultant Fe0@FexOy-Fe(II) structure and was sustained by electrons (from cathode) and [H] via the in situ reduction of Fe(III) back to Fe(II). Meanwhile, the undesirable product NH4+ was efficiently oxidized to N2 by the active chlorine generated on the anode. This study not only clarifies the mechanism of enhanced nitrate reduction on nZVI via electrochemical regulation but also advances the technological coupling of nZVI reduction with electrochemistry.
中文翻译:

纳米级零价铁的电化学介导的硝酸盐还原:性质和机理。
将硝酸盐选择性还原成N2是有吸引力的,但在水处理领域仍然是一个艰巨的挑战。在这里,我们建立了一种流通式电化学系统,该系统中装有支持nZVI(nZVI @ D201)的聚合物珠粒,用于选择性还原硝酸盐。因此,在nZVI @ D201上,在电化学调节下,在至少60 h内,N2选择性高达95%时,可以实现高效的硝酸盐还原。否则,20小时后nZVI逐渐耗尽,产物主要是不希望的NH4 +。通过一系列比较实验,我们澄清了在电化学调节下nZVI硝酸盐还原的增加主要归因于电子(来自阴极)和活性氢([H]),而不是先前推测的H2。结合X射线衍射和X射线光电子能谱仪对硝酸盐还原过程中nZVI的表征,我们发现,电化学调节下的硝酸盐还原作用是由nZVI介导的,以及由此产生的Fe0 @ FexOy-Fe(II)结构并由电子维持(来自阴极)和[H]通过原位还原Fe(III)还原为Fe(II)。同时,不良产物NH4 +被阳极上产生的活性氯有效地氧化成N2。这项研究不仅阐明了通过电化学调节提高硝酸盐还原nZVI的机理,而且还促进了nZVI还原与电化学的技术耦合。我们发现,电化学调控下的硝酸盐还原是由nZVI以及所生成的Fe0 @ FexOy-Fe(II)结构介导的,并且通过电子(来自阴极)和[H]通过原位还原Fe(III)到铁(II)。同时,不良产物NH4 +被阳极上产生的活性氯有效地氧化成N2。这项研究不仅阐明了通过电化学调节提高硝酸盐还原nZVI的机理,而且还促进了nZVI还原与电化学的技术耦合。我们发现,电化学调控下的硝酸盐还原是由nZVI以及所生成的Fe0 @ FexOy-Fe(II)结构介导的,并且通过电子(来自阴极)和[H]通过原位还原Fe(III)到铁(II)。同时,不良产物NH4 +被阳极上产生的活性氯有效地氧化成N2。这项研究不仅阐明了通过电化学调节提高硝酸盐还原nZVI的机理,而且还促进了nZVI还原与电化学的技术耦合。
更新日期:2020-02-10
中文翻译:

纳米级零价铁的电化学介导的硝酸盐还原:性质和机理。
将硝酸盐选择性还原成N2是有吸引力的,但在水处理领域仍然是一个艰巨的挑战。在这里,我们建立了一种流通式电化学系统,该系统中装有支持nZVI(nZVI @ D201)的聚合物珠粒,用于选择性还原硝酸盐。因此,在nZVI @ D201上,在电化学调节下,在至少60 h内,N2选择性高达95%时,可以实现高效的硝酸盐还原。否则,20小时后nZVI逐渐耗尽,产物主要是不希望的NH4 +。通过一系列比较实验,我们澄清了在电化学调节下nZVI硝酸盐还原的增加主要归因于电子(来自阴极)和活性氢([H]),而不是先前推测的H2。结合X射线衍射和X射线光电子能谱仪对硝酸盐还原过程中nZVI的表征,我们发现,电化学调节下的硝酸盐还原作用是由nZVI介导的,以及由此产生的Fe0 @ FexOy-Fe(II)结构并由电子维持(来自阴极)和[H]通过原位还原Fe(III)还原为Fe(II)。同时,不良产物NH4 +被阳极上产生的活性氯有效地氧化成N2。这项研究不仅阐明了通过电化学调节提高硝酸盐还原nZVI的机理,而且还促进了nZVI还原与电化学的技术耦合。我们发现,电化学调控下的硝酸盐还原是由nZVI以及所生成的Fe0 @ FexOy-Fe(II)结构介导的,并且通过电子(来自阴极)和[H]通过原位还原Fe(III)到铁(II)。同时,不良产物NH4 +被阳极上产生的活性氯有效地氧化成N2。这项研究不仅阐明了通过电化学调节提高硝酸盐还原nZVI的机理,而且还促进了nZVI还原与电化学的技术耦合。我们发现,电化学调控下的硝酸盐还原是由nZVI以及所生成的Fe0 @ FexOy-Fe(II)结构介导的,并且通过电子(来自阴极)和[H]通过原位还原Fe(III)到铁(II)。同时,不良产物NH4 +被阳极上产生的活性氯有效地氧化成N2。这项研究不仅阐明了通过电化学调节提高硝酸盐还原nZVI的机理,而且还促进了nZVI还原与电化学的技术耦合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号