Energy Storage Materials ( IF 20.4 ) Pub Date : 2020-02-07 , DOI: 10.1016/j.ensm.2020.02.004 Lin Tao , Yunpeng Yang , Huanlei Wang , Yulong Zheng , Hongchang Hao , Wenping Song , Jing Shi , Minghua Huang , David Mitlin
|
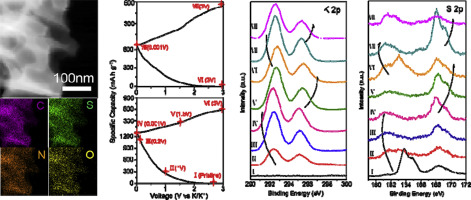
Combined sulfur and nitrogen (S = 12.9 at.%, N = 9.9 at.%) rich carbons are synthesized for potassium ion anode applications. The low-surface-area carbons (56 m2 g-1) have sulfur covalently bonded to the structure, with minimum unbound "free" sulfur. This allows for exceptional rate capability and stability: Capacities of 437, 234 and 72 mAh g-1 are achieved at 0.1, 1 and 10 A g-1, with 75% retention at 2 A g-1 after 3000 cycles. These are among the most favorable capacity-cyclability combinations reported in potassium ion battery carbon literature. As a proof of principle, the carbons are incorporated into a potassium ion capacitor with state-of-the-art energy and power (e.g. 110 W h kg-1 at 244 W kg -1). According to XPS analysis, the reaction of nitrogen with K+ is distinct from that of K+ with sulfur. The N and N-O moieties undergo a series of complex multi-voltage reactions that result in both reversible and irreversible changes to their structure. The K-S reactions involve a combination of reversible adsorption and reversible formation of sulfides, thiosulfate and sulfate. GITT and EIS analysis indicate that incorporation of S into the N-rich carbon increases the K+ solid-state diffusion coefficient by factors ranging from ∼3 to 8, depending on the voltage. The diffusivities are asymmetric with charging vs. discharging, signifying distinct reaction pathways. The covalently bound sulfur also has a positive influence on the solid electrolyte interphase (SEI) formation, at early and at prolonged cycling.
中文翻译:

富硫氮碳作为稳定的高容量钾离子电池负极:性能和存储机理
合成了富含硫和氮(S = 12.9 at。%,N = 9.9 at。%)的碳,用于钾离子阳极应用。低表面积碳(56 m 2 g -1)具有与结构共价键合的硫,而未结合的“游离”硫最少。这提供了出色的速率能力和稳定性:在0.1、1和10 A g -1时可实现437、234和72 mAh g -1的容量,在3000次循环后在2 A g -1时可保留75%的容量。这些是钾离子电池碳文献中报道的最有利的容量-循环能力组合。作为原理上的证明,碳被结合到具有最新能量和功率的钾离子电容器中(例如110 W h kg -1在244 W kg -1)。根据XPS分析,氮与K +的反应不同于K +与硫的反应。N和NO部分会经历一系列复杂的多电压反应,从而导致其结构可逆和不可逆变化。KS反应包括可逆吸附和可逆形成硫化物,硫代硫酸盐和硫酸盐的组合。GITT和EIS分析表明,根据电压的不同,将S掺入富氮碳中可使K +固态扩散系数提高约3至8。的扩散系数是不对称的充电与放电,表示不同的反应途径。在早期和长时间循环中,共价键合的硫对固体电解质中间相(SEI)的形成也具有积极影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号