当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unraveling the RNA Binding Properties of the Iron-Sulfur Zinc Finger Protein CPSF30.
Biochemistry ( IF 2.9 ) Pub Date : 2020-02-06 , DOI: 10.1021/acs.biochem.9b01065 Jordan D Pritts 1 , Matthew S Hursey 1 , Jamie L Michalek 1 , Sharon Batelu 2 , Timothy L Stemmler 2 , Sarah L J Michel 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-02-06 , DOI: 10.1021/acs.biochem.9b01065 Jordan D Pritts 1 , Matthew S Hursey 1 , Jamie L Michalek 1 , Sharon Batelu 2 , Timothy L Stemmler 2 , Sarah L J Michel 1
Affiliation
|
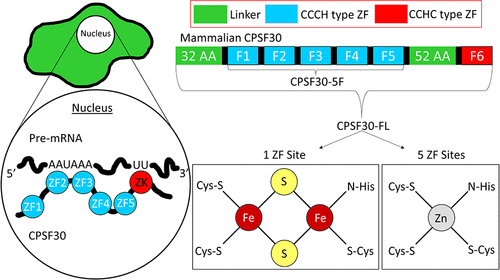
Cleavage and polyadenylation specificity factor 30 (CPSF30) is a "zinc finger" protein that plays a crucial role in the transition of pre-mRNA to RNA. CPSF30 contains five conserved CCCH domains and a CCHC "zinc knuckle" domain. CPSF30 activity is critical for pre-mRNA processing. A truncated form of the protein, in which only the CCCH domains are present, has been shown to specifically bind AU-rich pre-mRNA targets; however, the RNA binding and recognition properties of full-length CPSF30 are not known. Herein, we report the isolation and biochemical characterization of full-length CPSF30. We report that CPSF30 contains one 2Fe-2S cluster in addition to five zinc ions, as measured by inductively coupled plasma mass spectrometry, ultraviolet-visible spectroscopy, and X-ray absorption spectroscopy. Utilizing fluorescence anisotropy RNA binding assays, we show that full-length CPSF30 has high binding affinity for two types of pre-mRNA targets, AAUAAA and polyU, both of which are conserved sequence motifs present in the majority of pre-mRNAs. Binding to the AAUAAA motif requires that the five CCCH domains of CPSF30 be present, whereas binding to polyU sequences requires the entire, full-length CPSF30. These findings implicate the CCHC "zinc knuckle" present in the full-length protein as being critical for mediating polyU binding. We also report that truncated forms of the protein, containing either just two CCCH domains (ZF2 and ZF3) or the CCHC "zinc knuckle" domain, do not exhibit any RNA binding, indicating that CPSF30/RNA binding requires several ZF (and/or Fe-S cluster) domains working in concert to mediate RNA recognition.
中文翻译:

揭示铁硫锌指蛋白 CPSF30 的 RNA 结合特性。
切割和聚腺苷酸化特异性因子 30 (CPSF30) 是一种“锌指”蛋白,在前体 mRNA 到 RNA 的转变中起关键作用。CPSF30 包含五个保守的 CCCH 域和一个 CCHC“锌指关节”域。CPSF30 活性对于前体 mRNA 加工至关重要。一种截短形式的蛋白质,其中仅存在 CCCH 结构域,已显示特异性结合富含 AU 的前 mRNA 靶标;然而,全长 CPSF30 的 RNA 结合和识别特性尚不清楚。在此,我们报告了全长 CPSF30 的分离和生化表征。我们报告说,通过电感耦合等离子体质谱、紫外-可见光谱和 X 射线吸收光谱测量,CPSF30 除了五个锌离子外还包含一个 2Fe-2S 簇。利用荧光各向异性 RNA 结合测定,我们表明全长 CPSF30 对两种类型的前 mRNA 靶标 AAUAAA 和 polyU 具有高结合亲和力,这两种类型都是存在于大多数前 mRNA 中的保守序列基序。与 AAUAAA 基序的结合需要存在 CPSF30 的五个 CCCH 域,而与 polyU 序列的结合需要完整的全长 CPSF30。这些发现表明全长蛋白质中存在的 CCHC“锌指关节”对于介导 polyU 结合至关重要。我们还报告说,蛋白质的截短形式,仅包含两个 CCCH 域(ZF2 和 ZF3)或 CCHC“锌指”域,不表现出任何 RNA 结合,
更新日期:2020-02-20
中文翻译:

揭示铁硫锌指蛋白 CPSF30 的 RNA 结合特性。
切割和聚腺苷酸化特异性因子 30 (CPSF30) 是一种“锌指”蛋白,在前体 mRNA 到 RNA 的转变中起关键作用。CPSF30 包含五个保守的 CCCH 域和一个 CCHC“锌指关节”域。CPSF30 活性对于前体 mRNA 加工至关重要。一种截短形式的蛋白质,其中仅存在 CCCH 结构域,已显示特异性结合富含 AU 的前 mRNA 靶标;然而,全长 CPSF30 的 RNA 结合和识别特性尚不清楚。在此,我们报告了全长 CPSF30 的分离和生化表征。我们报告说,通过电感耦合等离子体质谱、紫外-可见光谱和 X 射线吸收光谱测量,CPSF30 除了五个锌离子外还包含一个 2Fe-2S 簇。利用荧光各向异性 RNA 结合测定,我们表明全长 CPSF30 对两种类型的前 mRNA 靶标 AAUAAA 和 polyU 具有高结合亲和力,这两种类型都是存在于大多数前 mRNA 中的保守序列基序。与 AAUAAA 基序的结合需要存在 CPSF30 的五个 CCCH 域,而与 polyU 序列的结合需要完整的全长 CPSF30。这些发现表明全长蛋白质中存在的 CCHC“锌指关节”对于介导 polyU 结合至关重要。我们还报告说,蛋白质的截短形式,仅包含两个 CCCH 域(ZF2 和 ZF3)或 CCHC“锌指”域,不表现出任何 RNA 结合,



























 京公网安备 11010802027423号
京公网安备 11010802027423号