Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial.
The Lancet ( IF 168.9 ) Pub Date : 2020-02-08 , DOI: 10.1016/s0140-6736(19)33161-7 Christopher T Ritchlin 1 , Arthur Kavanaugh 2 , Joseph F Merola 3 , Georg Schett 4 , Jose U Scher 5 , Richard B Warren 6 , Alice B Gottlieb 7 , Deepak Assudani 8 , Kathy Bedford-Rice 9 , Jason Coarse 9 , Barbara Ink 8 , Iain B McInnes 10
The Lancet ( IF 168.9 ) Pub Date : 2020-02-08 , DOI: 10.1016/s0140-6736(19)33161-7 Christopher T Ritchlin 1 , Arthur Kavanaugh 2 , Joseph F Merola 3 , Georg Schett 4 , Jose U Scher 5 , Richard B Warren 6 , Alice B Gottlieb 7 , Deepak Assudani 8 , Kathy Bedford-Rice 9 , Jason Coarse 9 , Barbara Ink 8 , Iain B McInnes 10
Affiliation
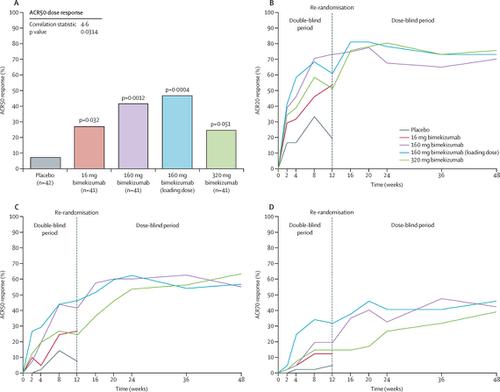
|
BACKGROUND
Dual neutralisation of interleukin 17A (IL17A) and interleukin 17F (IL17F) is a potential novel therapeutic approach in psoriatic arthritis. We assessed bimekizumab, a monoclonal antibody that selectively neutralises IL17A and IL17F, in patients with active psoriatic arthritis.
METHODS
BE ACTIVE was a randomised, double-blind, placebo-controlled, dose-ranging phase 2b study done at 41 sites in the Czech Republic, Germany, Hungary, Poland, Russia, and the USA. Eligible patients aged 18 years or older with active adult-onset psoriatic arthritis and symptoms for at least 6 months were randomly assigned (1:1:1:1:1) to placebo, 16 mg bimekizumab, 160 mg bimekizumab, 160 mg bimekizumab with a one-off 320 mg loading dose, or 320 mg bimekizumab, which were administered as subcutaneous injections every 4 weeks for 12 weeks. After 12 weeks, patients assigned to the placebo and 16 mg bimekizumab groups were randomly reassigned (1:1) to either 160 mg or 320 mg bimekizumab, and all other patients remained on their originally assigned initial dose up to 48 weeks. Both participants and researchers were blinded to treatment allocation in the first 12 weeks, and blinded to the dose of bimekizumab thereafter. The primary endpoint was the proportion of patients with at least 50% improvement in the American College of Rheumatology response criteria at week 12, which was assessed in all patients who received at least one dose of study treatment and had a valid measurement of the primary efficacy endpoint at baseline. The trial, including all follow-up, has been completed. This trial is registered with ClinicalTrials.gov, NCT02969525.
FINDINGS
Between Oct 27, 2016, and July 16, 2018, 308 patients were screened, and 206 were randomly assigned: 42 to the placebo group, and 41 each to the four bimekizumab groups. At 12 weeks, compared with the placebo group, significantly more patients in the 16 mg bimekizumab (odds ratio [OR] 4·2 [95% CI 1·1-15·2]; p=0·032), 160 mg bimekizumab (8·1 [2·3-28·7]; p=0·0012), and 160 mg (loading dose) bimekizumab (9·7 [2·7-34·3]; p=0·0004) groups achieved an ACR50 response. At 12 weeks, 24 (57%) of 42 patients in the placebo group and 68 (41%) of the 164 patients in the bimekizumab groups reported treatment-emergent adverse events. Most of these adverse events were mild or moderate. Serious treatment-emergent adverse events occurred in nine patients, eight of whom were receiving bimekizumab. No deaths or cases of inflammatory bowel disease were reported.
INTERPRETATION
Bimekizumab doses of 16 mg and 160 mg (with or without a 320 mg loading dose) were associated with significant improvements in ACR50 compared with placebo, with an acceptable safety profile. Our results support phase 3 investigation of bimekizumab as a treatment for psoriatic arthritis.
FUNDING
UCB Pharma.
中文翻译:

活动性银屑病关节炎患者的比米珠单抗:一项为期48周,随机,双盲,安慰剂对照,剂量范围2b期试验的结果。
背景技术白介素17A(IL17A)和白介素17F(IL17F)的双重中和是银屑病关节炎中潜在的新型治疗方法。我们评估了患有活动性银屑病关节炎的患者bimekizumab(一种选择性中和IL17A和IL17F的单克隆抗体)。“活跃的方法”是在捷克共和国,德国,匈牙利,波兰,俄罗斯和美国的41个地点进行的随机,双盲,安慰剂对照,剂量范围2b期研究。年龄在18岁或以上且患有活动性成人发作性银屑病关节炎且症状持续至少6个月的合格患者被随机分配(1:1:1:1:1)安慰剂,16 mg比米珠单抗,160 mg比米珠单抗,160 mg比米珠单抗与一次性320毫克负荷剂量或320毫克bimekizumab,每4周一次皮下注射,持续12周。12周后 分配给安慰剂组和16 mg比美珠单抗组的患者被随机(1:1)分配给160 mg或320 mg比美珠单抗,所有其他患者保持最初分配的初始剂量长达48周。参与者和研究人员在开始的12周内都对分配的药物不知情,之后对bimekizumab的剂量不知情。主要终点指标是第12周时美国风湿病学会缓解标准改善至少50%的患者比例,该比例在接受至少一剂研究治疗剂量并已有效评估主要疗效的所有患者中进行评估基线处的终点。包括所有后续行动在内的试验已经完成。该试验已在ClinicalTrials.gov注册,编号为NCT02969525。发现在2016年10月27日至2018年7月16日之间,筛选了308例患者,并随机分配了206例患者:安慰剂组42例,比美昔单抗4例组41例。与安慰剂组相比,在第12周时,比格珠单抗160 mg(比值比[OR] 4·2 [95%CI 1·1-15·2]; p = 0·032)明显多于16 mg比美珠单抗(8·1 [2·3-28·7]; p = 0·0012)和比美单抗160毫克(负荷剂量)(9·7 [2·7-34·3]; p = 0·0004)组达到了ACR50响应。在第12周时,安慰剂组的42位患者中有24位(57%),比美珠单抗组的164位患者中有68位(41%)报告了紧急治疗事件。这些不良事件大多数为轻度或中度。9名患者发生了严重的治疗紧急不良事件,其中8名患者接受了bimekizumab治疗。没有死亡或炎症性肠病病例的报道。解释与安慰剂相比,比美珠单抗剂量分别为16 mg和160 mg(有或没有320 mg负荷剂量)与ACR50的显着改善有关,且安全性可接受。我们的结果支持bimekizumab作为银屑病关节炎治疗的3期研究。资助UCB Pharma。
更新日期:2020-02-06
中文翻译:

活动性银屑病关节炎患者的比米珠单抗:一项为期48周,随机,双盲,安慰剂对照,剂量范围2b期试验的结果。
背景技术白介素17A(IL17A)和白介素17F(IL17F)的双重中和是银屑病关节炎中潜在的新型治疗方法。我们评估了患有活动性银屑病关节炎的患者bimekizumab(一种选择性中和IL17A和IL17F的单克隆抗体)。“活跃的方法”是在捷克共和国,德国,匈牙利,波兰,俄罗斯和美国的41个地点进行的随机,双盲,安慰剂对照,剂量范围2b期研究。年龄在18岁或以上且患有活动性成人发作性银屑病关节炎且症状持续至少6个月的合格患者被随机分配(1:1:1:1:1)安慰剂,16 mg比米珠单抗,160 mg比米珠单抗,160 mg比米珠单抗与一次性320毫克负荷剂量或320毫克bimekizumab,每4周一次皮下注射,持续12周。12周后 分配给安慰剂组和16 mg比美珠单抗组的患者被随机(1:1)分配给160 mg或320 mg比美珠单抗,所有其他患者保持最初分配的初始剂量长达48周。参与者和研究人员在开始的12周内都对分配的药物不知情,之后对bimekizumab的剂量不知情。主要终点指标是第12周时美国风湿病学会缓解标准改善至少50%的患者比例,该比例在接受至少一剂研究治疗剂量并已有效评估主要疗效的所有患者中进行评估基线处的终点。包括所有后续行动在内的试验已经完成。该试验已在ClinicalTrials.gov注册,编号为NCT02969525。发现在2016年10月27日至2018年7月16日之间,筛选了308例患者,并随机分配了206例患者:安慰剂组42例,比美昔单抗4例组41例。与安慰剂组相比,在第12周时,比格珠单抗160 mg(比值比[OR] 4·2 [95%CI 1·1-15·2]; p = 0·032)明显多于16 mg比美珠单抗(8·1 [2·3-28·7]; p = 0·0012)和比美单抗160毫克(负荷剂量)(9·7 [2·7-34·3]; p = 0·0004)组达到了ACR50响应。在第12周时,安慰剂组的42位患者中有24位(57%),比美珠单抗组的164位患者中有68位(41%)报告了紧急治疗事件。这些不良事件大多数为轻度或中度。9名患者发生了严重的治疗紧急不良事件,其中8名患者接受了bimekizumab治疗。没有死亡或炎症性肠病病例的报道。解释与安慰剂相比,比美珠单抗剂量分别为16 mg和160 mg(有或没有320 mg负荷剂量)与ACR50的显着改善有关,且安全性可接受。我们的结果支持bimekizumab作为银屑病关节炎治疗的3期研究。资助UCB Pharma。


























 京公网安备 11010802027423号
京公网安备 11010802027423号