当前位置:
X-MOL 学术
›
Nat. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Proteomimetics as protein-inspired scaffolds with defined tertiary folding patterns.
Nature Chemistry ( IF 21.8 ) Pub Date : 2020-02-06 , DOI: 10.1038/s41557-020-0420-9 W Seth Horne 1 , Tom N Grossmann 2
Nature Chemistry ( IF 21.8 ) Pub Date : 2020-02-06 , DOI: 10.1038/s41557-020-0420-9 W Seth Horne 1 , Tom N Grossmann 2
Affiliation
|
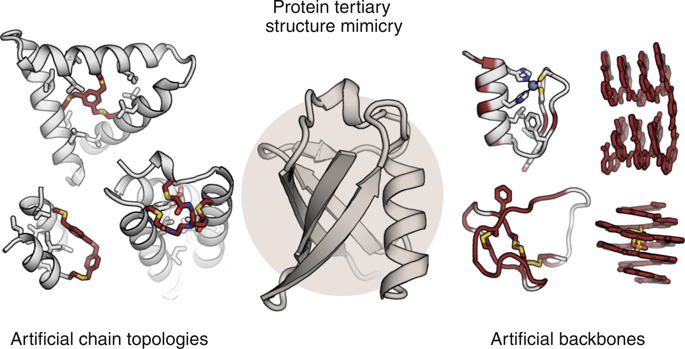
Proteins have evolved as a variable platform that provides access to molecules with diverse shapes, sizes and functions. These features have inspired chemists for decades to seek artificial mimetics of proteins with improved or novel properties. Such work has focused primarily on small protein fragments, often isolated secondary structures; however, there has lately been a growing interest in the design of artificial molecules that mimic larger, more complex tertiary folds. In this Perspective, we define these agents as 'proteomimetics' and discuss the recent advances in the field. Proteomimetics can be divided into three categories: protein domains with side-chain functionality that alters the native linear-chain topology; protein domains in which the chemical composition of the polypeptide backbone has been partially altered; and protein-like folded architectures that are composed entirely of non-natural monomer units. We give an overview of these proteomimetic approaches and outline remaining challenges facing the field.
中文翻译:

蛋白质模拟物是具有启发性的三级折叠模式的蛋白质启发的支架。
蛋白质已经发展成为一个可变的平台,可以访问具有各种形状,大小和功能的分子。这些特征激发了化学家数十年来寻求具有改进或新颖特性的蛋白质的人工模拟物。这些工作主要集中在小的蛋白质片段上,通常是孤立的二级结构。然而,近来人们对模拟更大,更复杂的三级折叠的人工分子的设计越来越感兴趣。在此观点中,我们将这些代理定义为“蛋白质模拟”,并讨论该领域的最新进展。蛋白质组学可分为三类:具有改变天然线性链拓扑结构的侧链功能的蛋白质结构域;蛋白质结构域,其中多肽主链的化学组成已部分改变;以及完全由非天然单体单元组成的类似蛋白质的折叠结构。我们对这些蛋白质组学方法进行了概述,并概述了该领域仍面临的挑战。
更新日期:2020-02-06
中文翻译:

蛋白质模拟物是具有启发性的三级折叠模式的蛋白质启发的支架。
蛋白质已经发展成为一个可变的平台,可以访问具有各种形状,大小和功能的分子。这些特征激发了化学家数十年来寻求具有改进或新颖特性的蛋白质的人工模拟物。这些工作主要集中在小的蛋白质片段上,通常是孤立的二级结构。然而,近来人们对模拟更大,更复杂的三级折叠的人工分子的设计越来越感兴趣。在此观点中,我们将这些代理定义为“蛋白质模拟”,并讨论该领域的最新进展。蛋白质组学可分为三类:具有改变天然线性链拓扑结构的侧链功能的蛋白质结构域;蛋白质结构域,其中多肽主链的化学组成已部分改变;以及完全由非天然单体单元组成的类似蛋白质的折叠结构。我们对这些蛋白质组学方法进行了概述,并概述了该领域仍面临的挑战。


























 京公网安备 11010802027423号
京公网安备 11010802027423号