当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
From the Amelioration of a NADP+‐dependent Formate Dehydrogenase to the Discovery of a New Enzyme: Round Trip from Theory to Practice
ChemCatChem ( IF 4.5 ) Pub Date : 2020-03-19 , DOI: 10.1002/cctc.201902089 Marina Simona Robescu 1 , Rudy Rubini 1 , Elisa Beneventi 1 , Michele Tavanti 1 , Chiara Lonigro 1, 2 , Francesca Zito 2 , Francesco Filippini 1 , Laura Cendron 1 , Elisabetta Bergantino 1
ChemCatChem ( IF 4.5 ) Pub Date : 2020-03-19 , DOI: 10.1002/cctc.201902089 Marina Simona Robescu 1 , Rudy Rubini 1 , Elisa Beneventi 1 , Michele Tavanti 1 , Chiara Lonigro 1, 2 , Francesca Zito 2 , Francesco Filippini 1 , Laura Cendron 1 , Elisabetta Bergantino 1
Affiliation
|
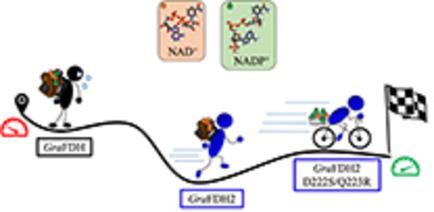
NADP+‐dependent formate dehydrogenases (FDHs) are biotechnologically relevant enzymes for cofactors regeneration in industrial processes employing redox biocatalysts. Their effective applicability is however hampered by the low cofactor and substrate affinities of the few enzymes described so far. After different efforts to ameliorate the previously studied GraFDH from the acidobacterium Granulicella mallensis MP5ACTX8, an enzyme having double (NAD+ and NADP+) cofactor specificity, we started over our search with the advantage of hindsight. We identified and characterized GraFDH2, a novel highly active FDH, which proved to be a good NAD+‐dependent catalyst. A rational engineering approach permitted to switch its cofactor specificity, producing an enzyme variant that displays a 10‐fold activity improvement over the wild‐type enzyme with NADP+. Such variant resulted to be one of the best performing enzyme among the NADP+‐dependent FDHs reported so far in terms of catalytic performance.
中文翻译:

从改善NADP +依赖的甲酰胺脱氢酶到发现新酶:从理论到实践的往返
NADP +依赖的甲酸脱氢酶(FDHs)是生物技术上相关的酶,可在使用氧化还原生物催化剂的工业过程中辅助因子的再生。然而,迄今为止描述的几种酶的低辅因子和底物亲和力阻碍了它们的有效适用性。经过不同的努力来改善先前研究的来自酸杆菌Granulicella mallensis MP5ACTX8的Gra FDH,这是一种具有双倍(NAD +和NADP +)辅因子特异性的酶,我们从事后观察的角度开始了我们的搜索。我们鉴定并表征了Gra FDH2,这是一种新型的高活性FDH,被证明是一种很好的NAD +依赖的催化剂。合理的工程方法允许切换其辅因子特异性,从而产生一种酶变体,该酶变体的活性比使用NADP +的野生型酶提高了10倍。就催化性能而言,迄今为止,这种变体是NADP +依赖性FDH中表现最好的酶之一。
更新日期:2020-03-19
中文翻译:

从改善NADP +依赖的甲酰胺脱氢酶到发现新酶:从理论到实践的往返
NADP +依赖的甲酸脱氢酶(FDHs)是生物技术上相关的酶,可在使用氧化还原生物催化剂的工业过程中辅助因子的再生。然而,迄今为止描述的几种酶的低辅因子和底物亲和力阻碍了它们的有效适用性。经过不同的努力来改善先前研究的来自酸杆菌Granulicella mallensis MP5ACTX8的Gra FDH,这是一种具有双倍(NAD +和NADP +)辅因子特异性的酶,我们从事后观察的角度开始了我们的搜索。我们鉴定并表征了Gra FDH2,这是一种新型的高活性FDH,被证明是一种很好的NAD +依赖的催化剂。合理的工程方法允许切换其辅因子特异性,从而产生一种酶变体,该酶变体的活性比使用NADP +的野生型酶提高了10倍。就催化性能而言,迄今为止,这种变体是NADP +依赖性FDH中表现最好的酶之一。


























 京公网安备 11010802027423号
京公网安备 11010802027423号