当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Contaminant Degradation by •OH during Sediment Oxygenation: Dependence on Fe(II) Species.
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-02-14 , DOI: 10.1021/acs.est.9b04870 Wenjing Xie 1 , Songhu Yuan 1 , Man Tong 1 , Sicong Ma 1 , Wenjuan Liao 1 , Na Zhang 1 , Chunmei Chen 2
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-02-14 , DOI: 10.1021/acs.est.9b04870 Wenjing Xie 1 , Songhu Yuan 1 , Man Tong 1 , Sicong Ma 1 , Wenjuan Liao 1 , Na Zhang 1 , Chunmei Chen 2
Affiliation
|
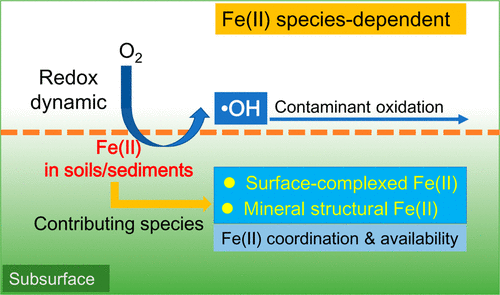
It has been documented that contaminants could be degraded by hydroxyl radicals (•OH) produced upon oxygenation of Fe(II)-bearing sediments. However, the dependence of contaminant degradation on sediment characteristics, particularly Fe(II) species, remains elusive. Here we assessed the impact of the abundance of Fe(II) species in sediments on contaminant degradation by •OH during oxygenation. Three natural sediments with different Fe(II) contents and species were oxygenated. During 10 h oxygenation of 200 g/L sediment suspension, 2 mg/L phenol was negligibly degraded for sandbeach sediment (Fe(II): 9.11 mg/g), but was degraded by 41% and 52% for lakeshore (Fe(II): 9.81 mg/g) and farmland (Fe(II): 19.05 mg/g) sediments, respectively. •OH produced from Fe(II) oxygenation was the key reactive oxidant. Sequential extractions, X-ray diffraction, Mössbauer, and X-ray absorption spectroscopy suggest that surface-adsorbed Fe(II) and mineral structural Fe(II) contributed predominantly to •OH production and phenol degradation. Control experiments with specific Fe(II) species and coordination structure analysis collectively suggest the likely rule that Fe(II) oxidation rate and its competition for •OH increase with the increase in electron-donating ability of the atoms (i.e., O) complexed to Fe(II), while the •OH yield decreases accordingly. The Fe(II) species with a moderate oxidation rate and •OH yield is most favorable for contaminant degradation.
中文翻译:

沉积物氧合过程中•OH造成的污染物降解:取决于Fe(II)物种。
已有文献证明,在含Fe(II)的沉积物氧合时,产生的羟基自由基(•OH)会降解污染物。但是,污染物降解对沉积物特征,尤其是Fe(II)物种的依赖性仍然难以捉摸。在这里,我们评估了沉积物中大量Fe(II)物种对氧合作用过程中•OH降解污染物的影响。三种具有不同Fe(II)含量和种类的天然沉积物被氧化。在200 g / L沉积物悬浮液的10小时充氧过程中,沙海滩沉积物(Fe(II):9.11 mg / g)的2 mg / L苯酚可忽略不计,而湖岸(Fe(II) ):9.81 mg / g)和农田(Fe(II):19.05 mg / g)沉积物。Fe(II)氧化生成的•OH是关键的反应氧化剂。顺序提取,X射线衍射,Mössbauer和X射线吸收光谱表明,表面吸附的Fe(II)和矿物结构的Fe(II)对•OH的产生和苯酚的降解起主要作用。用特定的Fe(II)物种进行的控制实验和配位结构分析共同表明,可能的规律是Fe(II)的氧化速率及其对•OH的竞争随着络合到Fe(II),而•OH收率相应降低。具有中等氧化速率和•OH收率的Fe(II)物种最有利于污染物降解。用特定的Fe(II)物种进行的控制实验和配位结构分析共同表明,可能的规律是Fe(II)的氧化速率及其对•OH的竞争随着络合到原子上的原子(即O)的供电子能力的增加而增加。 Fe(II),而•OH收率相应降低。具有中等氧化速率和•OH收率的Fe(II)物种最有利于污染物降解。用特定的Fe(II)物种进行的控制实验和配位结构分析共同表明,可能的规律是Fe(II)的氧化速率及其对•OH的竞争随着络合到原子上的原子(即O)的供电子能力的增加而增加。 Fe(II),而•OH收率相应降低。具有中等氧化速率和•OH收率的Fe(II)物种最有利于污染物降解。
更新日期:2020-02-17
中文翻译:

沉积物氧合过程中•OH造成的污染物降解:取决于Fe(II)物种。
已有文献证明,在含Fe(II)的沉积物氧合时,产生的羟基自由基(•OH)会降解污染物。但是,污染物降解对沉积物特征,尤其是Fe(II)物种的依赖性仍然难以捉摸。在这里,我们评估了沉积物中大量Fe(II)物种对氧合作用过程中•OH降解污染物的影响。三种具有不同Fe(II)含量和种类的天然沉积物被氧化。在200 g / L沉积物悬浮液的10小时充氧过程中,沙海滩沉积物(Fe(II):9.11 mg / g)的2 mg / L苯酚可忽略不计,而湖岸(Fe(II) ):9.81 mg / g)和农田(Fe(II):19.05 mg / g)沉积物。Fe(II)氧化生成的•OH是关键的反应氧化剂。顺序提取,X射线衍射,Mössbauer和X射线吸收光谱表明,表面吸附的Fe(II)和矿物结构的Fe(II)对•OH的产生和苯酚的降解起主要作用。用特定的Fe(II)物种进行的控制实验和配位结构分析共同表明,可能的规律是Fe(II)的氧化速率及其对•OH的竞争随着络合到Fe(II),而•OH收率相应降低。具有中等氧化速率和•OH收率的Fe(II)物种最有利于污染物降解。用特定的Fe(II)物种进行的控制实验和配位结构分析共同表明,可能的规律是Fe(II)的氧化速率及其对•OH的竞争随着络合到原子上的原子(即O)的供电子能力的增加而增加。 Fe(II),而•OH收率相应降低。具有中等氧化速率和•OH收率的Fe(II)物种最有利于污染物降解。用特定的Fe(II)物种进行的控制实验和配位结构分析共同表明,可能的规律是Fe(II)的氧化速率及其对•OH的竞争随着络合到原子上的原子(即O)的供电子能力的增加而增加。 Fe(II),而•OH收率相应降低。具有中等氧化速率和•OH收率的Fe(II)物种最有利于污染物降解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号