当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Recent Advances in Electrochemical Systems for Selective Fluorination of Organic Compounds.
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-02-04 , DOI: 10.1021/acs.accounts.9b00520 Toshio Fuchigami 1 , Shinsuke Inagi 2, 3
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-02-04 , DOI: 10.1021/acs.accounts.9b00520 Toshio Fuchigami 1 , Shinsuke Inagi 2, 3
Affiliation
|
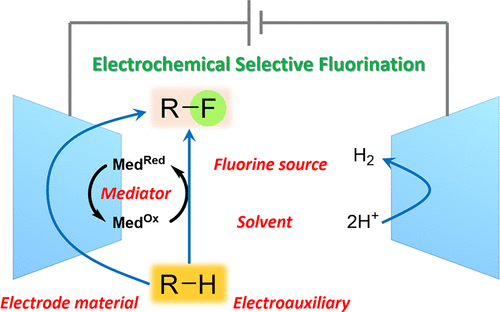
Organofluorine compounds are key materials applied in daily life because of their versatile utility as functional materials, pharmaceuticals, and agrochemicals. Development of the selective fluorination of organic molecules under safe conditions is therefore one of the most important subjects in modern synthetic organofluorine chemistry. Thus, various electrophilic fluorination reagents such as XeF2, (PhSO2)2NF (NFSI), Et2NSF3 (DAST), (MeOCH2CH2)2NSF3 (Deoxofluor), 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo-[2.2.2]octane bis(tetrafluoroborate) (Selectfluor), N-fluoropyridinium salts, and 4-tert-butyl-2,6-dimethylphenylsulfur trifluoride (Fluolead) have been developed for chemical fluorination to date and the development of new fluorinating reagents is still ongoing. Electrochemical synthesis has recently attracted much attention from the perspective of green sustainable chemistry because no hazardous reagents are required and scale-up is generally easy. Although electrochemical perfluorination of organic compounds using a nickel anode in anhydrous HF has been well-established to manufacture perfluoro-functional materials, electrochemical partial fluorination (selective electrochemical fluorination) has been underdeveloped due to the low nucleophilicity of fluoride ions and anode passivation, which interferes with electrolysis. Selective electrochemical fluorination can be commonly achieved in aprotic solvents containing fluoride ions to provide mostly mono- and difluorinated products. Electrolysis is conducted at constant potentials slightly higher than the first oxidation potential of a substrate. Constant current electrolysis is also effective for selective fluorination in many cases. Choice of the combination of a supporting fluoride salt and an electrolytic solvent is most important to accomplish efficient selective fluorination. In this Account, we focus on our recent work on the electrochemical mono- and difluorination of various organic compounds and their synthetic application. We first briefly explain our research background of electrochemical fluorination. Main factors such as the effects of fluoride salts as supporting electrolytes, electrolytic solvents, and anode materials on the selectivity and efficiency of fluorination are discussed. Next, effects of PEG oligomer additives enhancing the nucleophilicity of fluoride ions and organic solvent-free systems using poly(HF) salt ionic liquids as well as recyclable mediatory systems for electrochemical fluorination are described. The desulfurizative monofluorination of xanthate and gem-difluorination of benzothioate and dithioacetals are briefly mentioned. Regioselective anodic fluorination of various heterocyclic compounds having a phenylthio group as electroauxiliary and heterocycles containing sulfur and other heteroatoms are also described. In addition, a boryl group is shown to be a good leaving group for anodic fluorination. Moreover, electrochemically α,α-difluorinated phenylsulfides and phenylselenides are illustrated to be useful for photochemical C-H difluoromethylation of aromatic and heteroaromatic compounds. Finally, this Account also highlights highly diastereoselective fluorination of aliphatic heterocyclic and open-chain compounds, as well as new electrolytic fluorination methods using inorganic fluoride salts such as KF and CsF.
中文翻译:

选择性氟化有机化合物的电化学系统的最新进展。
有机氟化合物因其广泛用作功能材料,药物和农用化学品而成为日常生活中的关键材料。因此,在安全条件下开发有机分子的选择性氟化是现代合成有机氟化学中最重要的主题之一。因此,各种亲电子氟化试剂,例如XeF2,(PhSO2)2NF(NFSI),Et2NSF3(DAST),(MeOCH2CH2)2NSF3(Deoxofluor),1-氯甲基-4-氟-1,4-二氮杂双环-[2.2.2]迄今为止,辛烷烃双(四氟硼酸盐)(Selectfluor),N-氟吡啶鎓盐和4-叔丁基-2,6-二甲基苯基三氟化硫(Fluolead)已开发用于化学氟化,并且新的氟化试剂的开发仍在进行中。最近,从绿色可持续化学的角度出发,电化学合成备受关注,因为不需要有害试剂,而且通常易于放大。尽管已经建立了在无水HF中使用镍阳极对有机化合物进行电化学全氟化的方法来制造全氟功能材料,但是由于氟离子的亲核性低和阳极钝化,电化学部分氟化(选择性电化学氟化)的开发尚不完善。电解。选择性电化学氟化通常可以在含有氟离子的非质子溶剂中实现,从而主要提供单氟化和二氟化的产物。电解在恒定电势下进行,该恒定电势略高于衬底的第一氧化电势。在许多情况下,恒定电流电解对于选择性氟化也有效。选择支持的氟化物盐和电解质溶剂的组合对于实现有效的选择性氟化是最重要的。在这个帐户中,我们将重点放在各种有机化合物的电化学单氟化和二氟化及其合成应用方面的最新工作。我们首先简要解释我们的电化学氟化研究背景。讨论了诸如氟化盐作为支持电解质,电解质溶剂和阳极材料对氟化的选择性和效率的影响等主要因素。下一个,描述了PEG低聚物添加剂增强氟离子亲核性的效果,以及使用聚(HF)盐离子液体的无有机溶剂系统以及电化学氟化的可循环介质系统。简要提到了黄药的脱硫一氟化和苯硫基和二硫缩醛的宝石二氟化。还描述了各种具有苯硫基作为电助剂的杂环化合物的区域选择性阳极氟化,以及含有硫和其他杂原子的杂环。此外,硼烷基被证明是阳极氟化的良好离去基团。此外,说明了电化学上的α,α-二氟化苯硫和苯硒化物可用于芳族和杂芳族化合物的光化学CH二氟甲基化。最后,
更新日期:2020-02-06
中文翻译:

选择性氟化有机化合物的电化学系统的最新进展。
有机氟化合物因其广泛用作功能材料,药物和农用化学品而成为日常生活中的关键材料。因此,在安全条件下开发有机分子的选择性氟化是现代合成有机氟化学中最重要的主题之一。因此,各种亲电子氟化试剂,例如XeF2,(PhSO2)2NF(NFSI),Et2NSF3(DAST),(MeOCH2CH2)2NSF3(Deoxofluor),1-氯甲基-4-氟-1,4-二氮杂双环-[2.2.2]迄今为止,辛烷烃双(四氟硼酸盐)(Selectfluor),N-氟吡啶鎓盐和4-叔丁基-2,6-二甲基苯基三氟化硫(Fluolead)已开发用于化学氟化,并且新的氟化试剂的开发仍在进行中。最近,从绿色可持续化学的角度出发,电化学合成备受关注,因为不需要有害试剂,而且通常易于放大。尽管已经建立了在无水HF中使用镍阳极对有机化合物进行电化学全氟化的方法来制造全氟功能材料,但是由于氟离子的亲核性低和阳极钝化,电化学部分氟化(选择性电化学氟化)的开发尚不完善。电解。选择性电化学氟化通常可以在含有氟离子的非质子溶剂中实现,从而主要提供单氟化和二氟化的产物。电解在恒定电势下进行,该恒定电势略高于衬底的第一氧化电势。在许多情况下,恒定电流电解对于选择性氟化也有效。选择支持的氟化物盐和电解质溶剂的组合对于实现有效的选择性氟化是最重要的。在这个帐户中,我们将重点放在各种有机化合物的电化学单氟化和二氟化及其合成应用方面的最新工作。我们首先简要解释我们的电化学氟化研究背景。讨论了诸如氟化盐作为支持电解质,电解质溶剂和阳极材料对氟化的选择性和效率的影响等主要因素。下一个,描述了PEG低聚物添加剂增强氟离子亲核性的效果,以及使用聚(HF)盐离子液体的无有机溶剂系统以及电化学氟化的可循环介质系统。简要提到了黄药的脱硫一氟化和苯硫基和二硫缩醛的宝石二氟化。还描述了各种具有苯硫基作为电助剂的杂环化合物的区域选择性阳极氟化,以及含有硫和其他杂原子的杂环。此外,硼烷基被证明是阳极氟化的良好离去基团。此外,说明了电化学上的α,α-二氟化苯硫和苯硒化物可用于芳族和杂芳族化合物的光化学CH二氟甲基化。最后,


























 京公网安备 11010802027423号
京公网安备 11010802027423号