当前位置:
X-MOL 学术
›
Genes Brain Behav.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A mouse model for benign paroxysmal positional vertigo with genetic predisposition for displaced otoconia.
Genes, Brain and Behavior ( IF 2.5 ) Pub Date : 2020-01-02 , DOI: 10.1111/gbb.12635 Amiel A Dror 1, 2, 3 , Shahar Taiber 1 , Eyal Sela 2, 3 , Ophir Handzel 4, 5 , Karen B Avraham 1
Genes, Brain and Behavior ( IF 2.5 ) Pub Date : 2020-01-02 , DOI: 10.1111/gbb.12635 Amiel A Dror 1, 2, 3 , Shahar Taiber 1 , Eyal Sela 2, 3 , Ophir Handzel 4, 5 , Karen B Avraham 1
Affiliation
|
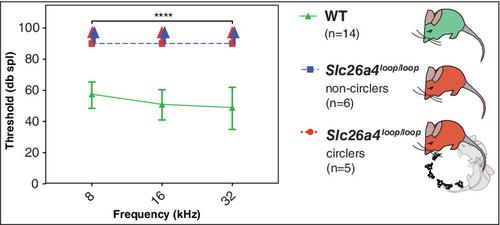
Abnormal formation of otoconia, the biominerals of the inner ear, results in balance disorders. The inertial mass of otoconia activates the underlying mechanosensory hair cells in response to change in head position primarily during linear and rotational acceleration. Otoconia associate exclusively with the two gravity receptors, the utricle and saccule. The cristae sensory epithelium is associated with an extracellular gelatinous matrix known as cupula, equivalent to otoconia. During head rotation, the inertia of endolymphatic fluids within the semicircular canals deflects the cupula of the corresponding crista and activates the underlying mechanosensory hair cells. It is believed that detached free‐floating otoconia particles travel ectopically to the semicircular canal and cristae and are the culprit for benign paroxysmal positional vertigo (BPPV). The Slc26a4 mouse mutant harbors a missense mutation in pendrin. This mutation leads to impaired transport activity of pendrin and to defects in otoconia composition and distribution. All Slc26a4
loop/loop homozygous mutant mice are profoundly deaf but show inconsistent vestibular deficiency. A panel of behavioral tests was utilized in order to generate a scoring method for vestibular function. A pathological finding of displaced otoconia was identified consistently in the inner ears of mutant mice with severe vestibular dysfunction. In this work, we present a mouse model with a genetic predisposition for ectopic otoconia with a clinical correlation to BPPV. This unique mouse model can serve as a platform for further investigation of BPPV pathophysiology, and for developing novel treatment approaches in a live animal model.
中文翻译:

良性阵发性位置性眩晕的小鼠模型,具有易感性耳畸形的遗传易感性。
内耳的生物矿物质耳垢的异常形成导致平衡失调。耳畸形的惯性质量主要在线性和旋转加速度期间响应于头部位置的变化而激活下面的机械感官毛细胞。Otoconia仅与两个重力受体,尿囊和球囊相关。ista感觉上皮与称为杯状的细胞外凝胶状基质相关,相当于耳菌病。在头部旋转过程中,半圆形管内的内淋巴液的惯性使相应的crista的吸盘偏斜,并激活下面的机械感觉毛细胞。据信分离的自由漂浮的耳垢颗粒异位到达半规管和cr,是良性阵发性位置性眩晕(BPPV)的罪魁祸首。的Slc26a4小鼠突变体在pendrin中具有错义突变。这种突变会导致Pendrin的运输活性受损,并导致耳垢的组成和分布出现缺陷。所有Slc26a4回路/回路 纯合突变小鼠严重失聪,但前庭缺乏显示不一致。利用一组行为测试来生成前庭功能的评分方法。在严重前庭功能障碍的突变小鼠的内耳中一致地发现了移位的耳垢的病理发现。在这项工作中,我们提出了具有异位耳垢的遗传易感性与BPPV临床相关性的小鼠模型。这种独特的小鼠模型可以作为进一步研究BPPV病理生理学以及开发活体动物模型中新颖治疗方法的平台。
更新日期:2020-01-02
中文翻译:

良性阵发性位置性眩晕的小鼠模型,具有易感性耳畸形的遗传易感性。
内耳的生物矿物质耳垢的异常形成导致平衡失调。耳畸形的惯性质量主要在线性和旋转加速度期间响应于头部位置的变化而激活下面的机械感官毛细胞。Otoconia仅与两个重力受体,尿囊和球囊相关。ista感觉上皮与称为杯状的细胞外凝胶状基质相关,相当于耳菌病。在头部旋转过程中,半圆形管内的内淋巴液的惯性使相应的crista的吸盘偏斜,并激活下面的机械感觉毛细胞。据信分离的自由漂浮的耳垢颗粒异位到达半规管和cr,是良性阵发性位置性眩晕(BPPV)的罪魁祸首。的Slc26a4小鼠突变体在pendrin中具有错义突变。这种突变会导致Pendrin的运输活性受损,并导致耳垢的组成和分布出现缺陷。所有Slc26a4回路/回路 纯合突变小鼠严重失聪,但前庭缺乏显示不一致。利用一组行为测试来生成前庭功能的评分方法。在严重前庭功能障碍的突变小鼠的内耳中一致地发现了移位的耳垢的病理发现。在这项工作中,我们提出了具有异位耳垢的遗传易感性与BPPV临床相关性的小鼠模型。这种独特的小鼠模型可以作为进一步研究BPPV病理生理学以及开发活体动物模型中新颖治疗方法的平台。



























 京公网安备 11010802027423号
京公网安备 11010802027423号