Electrocatalysis ( IF 3.1 ) Pub Date : 2019-07-22 , DOI: 10.1007/s12678-019-00546-1 Fernanda Juarez , Debora Salmazo , Paola Quaino , Wolfgang Schmickler
Water formation according to Had + OHad →H2O has recently been proposed as an intermediate step during hydrogen oxidation in alkaline solutions. Choosing Ni/Cu bimetallic surfaces as model catalysts, we have investigated the energetics and kinetics of this step in the form of a bifunctional mechanism. With the aid of density functional theory, we have identified several reaction paths on such surfaces with very low activation energies, suggesting that this step can be very fast. In all cases, the initial adsorption sites have both nickel and copper atoms as nearest neighbors. We suggest a strategy to find other bifunctional surfaces with good catalytic properties for this reaction.
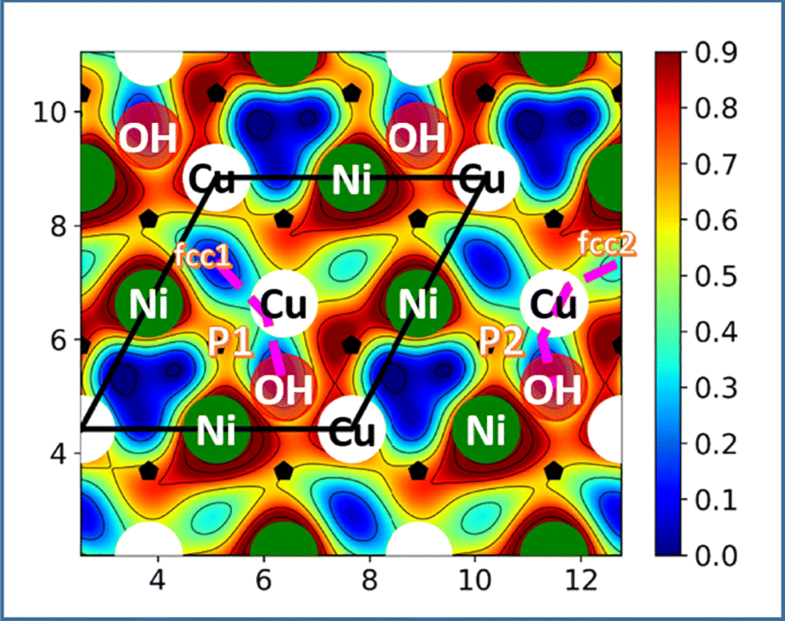
A bifunctional mechanism for the formation of water as an intermediate step for hydrogen oxidation is investigated by density functional theory. Using copper/nickel alloys of various compositions as an example, potential energy surfaces are calculated for various reaction paths. On some favorable sites the reaction may be too fast to be observed by electrochemical methods.
中文翻译:

碱性介质中的氢氧化:水形成的双功能机理
根据水的形成ħ一个d + O ħ一个d → ħ 2 O具有最近被提出作为在碱性溶液中氢的氧化过程中的中间步骤。选择Ni / Cu双金属表面作为模型催化剂,我们以双功能机理的形式研究了该步骤的能量和动力学。借助密度泛函理论,我们已经确定了在此类表面上具有极低活化能的多个反应路径,这表明该步骤可能非常快。在所有情况下,初始吸附位均具有镍和铜原子作为最近邻点。我们建议一种策略,以寻找其他具有良好催化性能的双功能表面进行此反应。

通过密度泛函理论研究了形成水作为氢氧化中间步骤的双功能机理。以各种组成的铜/镍合金为例,计算出各种反应路径的势能面。在某些有利的位置,反应可能太快而无法通过电化学方法观察到。

























 京公网安备 11010802027423号
京公网安备 11010802027423号