当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In vivo monitoring of remnant undifferentiated neural cells following human induced pluripotent stem cell-derived neural stem/progenitor cells transplantation.
STEM CELLS Translational Medicine ( IF 6 ) Pub Date : 2020-01-06 , DOI: 10.1002/sctm.19-0150 Yuji Tanimoto 1, 2, 3 , Tomoteru Yamasaki 3 , Narihito Nagoshi 2 , Yuichiro Nishiyama 2 , Satoshi Nori 2 , Soraya Nishimura 2 , Tsuyoshi Iida 2 , Masahiro Ozaki 2 , Osahiko Tsuji 2 , Bin Ji 4 , Ichio Aoki 5 , Masahiro Jinzaki 6 , Morio Matsumoto 2 , Yasuhisa Fujibayashi 3, 6 , Ming-Rong Zhang 3 , Masaya Nakamura 2 , Hideyuki Okano 1
STEM CELLS Translational Medicine ( IF 6 ) Pub Date : 2020-01-06 , DOI: 10.1002/sctm.19-0150 Yuji Tanimoto 1, 2, 3 , Tomoteru Yamasaki 3 , Narihito Nagoshi 2 , Yuichiro Nishiyama 2 , Satoshi Nori 2 , Soraya Nishimura 2 , Tsuyoshi Iida 2 , Masahiro Ozaki 2 , Osahiko Tsuji 2 , Bin Ji 4 , Ichio Aoki 5 , Masahiro Jinzaki 6 , Morio Matsumoto 2 , Yasuhisa Fujibayashi 3, 6 , Ming-Rong Zhang 3 , Masaya Nakamura 2 , Hideyuki Okano 1
Affiliation
|
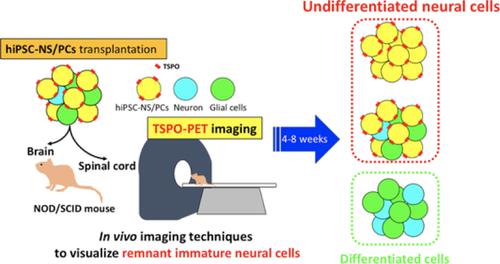
Transplantation of human‐induced pluripotent stem cell‐derived neural stem/progenitor cells (hiPSC‐NS/PCs) is a promising treatment for a variety of neuropathological conditions. Although previous reports have indicated the effectiveness of hiPSC‐NS/PCs transplantation into the injured spinal cord of rodents and nonhuman primates, long‐term observation of hiPSC‐NS/PCs post‐transplantation suggested some “unsafe” differentiation‐resistant properties, resulting in disordered overgrowth. These findings suggest that, even if “safe” NS/PCs are transplanted into the human central nervous system (CNS), the dynamics of cellular differentiation of stem cells should be noninvasively tracked to ensure safety. Positron emission tomography (PET) provides molecular‐functional information and helps to detect specific disease conditions. The current study was conducted to visualize Nestin (an NS/PC marker)‐positive undifferentiated neural cells in the CNS of immune‐deficient (nonobese diabetic‐severe combined immune‐deficient) mice after hiPSC‐NS/PCs transplantation with PET, using 18 kDa translocator protein (TSPO) ligands as labels. TSPO was recently found to be expressed in rodent NS/PCs, and its expression decreased with the progression of neuronal differentiation. We hypothesized that TSPO would also be present in hiPSC‐NS/PCs and expressed strongly in residual immature neural cells after transplantation. The results showed high levels of TSPO expression in immature hiPSC‐NS/PCs‐derived cells, and decreased TSPO expression as neural differentiation progressed in vitro. Furthermore, PET with [18F] FEDAC (a TSPO radioligand) was able to visualize the remnant undifferentiated hiPSC‐NS/PCs‐derived cells consisting of TSPO and Nestin+ cells in vivo. These findings suggest that PET with [18F] FEDAC could play a key role in the safe clinical application of CNS repair in regenerative medicine.
中文翻译:

人诱导多能干细胞衍生的神经干/祖细胞移植后残余未分化神经细胞的体内监测。
人诱导多能干细胞衍生的神经干/祖细胞(hiPSC-NS/PC)的移植是治疗多种神经病理性疾病的一种有前途的治疗方法。尽管之前的报道已经表明 hiPSC-NS/PC 移植到啮齿动物和非人灵长类动物受损脊髓中的有效性,但移植后 hiPSC-NS/PC 的长期观察表明存在一些“不安全”的抗分化特性,导致无序过度生长。这些发现表明,即使“安全”的 NS/PC 被移植到人类中枢神经系统 (CNS) 中,也应该无创地跟踪干细胞的细胞分化动态以确保安全。正电子发射断层扫描 (PET) 提供分子功能信息,有助于检测特定的疾病状况。目前的研究使用 18 种方法,对免疫缺陷(非肥胖糖尿病-严重联合免疫缺陷)小鼠的 hiPSC-NS/PC 移植后中枢神经系统中的 Nestin(一种 NS/PC 标记物)阳性未分化神经细胞进行可视化。 kDa 易位蛋白 (TSPO) 配体作为标记。最近发现TSPO在啮齿动物NS/PCs中表达,并且其表达随着神经元分化的进展而减少。我们假设 TSPO 也存在于 hiPSC-NS/PC 中,并在移植后残留的未成熟神经细胞中强烈表达。结果显示,未成熟的 hiPSC-NS/PC 衍生细胞中 TSPO 表达水平较高,并且随着体外神经分化的进展,TSPO 表达降低。此外,使用 [ 18 F] FEDAC(一种 TSPO 放射性配体)进行 PET 检测能够在体内观察到由 TSPO 和 Nestin +细胞组成的剩余未分化 hiPSC-NS/PC 衍生细胞。这些发现表明,具有[ 18 F] FEDAC的 PET可以在再生医学中中枢神经系统修复的安全临床应用中发挥关键作用。
更新日期:2020-01-06
中文翻译:

人诱导多能干细胞衍生的神经干/祖细胞移植后残余未分化神经细胞的体内监测。
人诱导多能干细胞衍生的神经干/祖细胞(hiPSC-NS/PC)的移植是治疗多种神经病理性疾病的一种有前途的治疗方法。尽管之前的报道已经表明 hiPSC-NS/PC 移植到啮齿动物和非人灵长类动物受损脊髓中的有效性,但移植后 hiPSC-NS/PC 的长期观察表明存在一些“不安全”的抗分化特性,导致无序过度生长。这些发现表明,即使“安全”的 NS/PC 被移植到人类中枢神经系统 (CNS) 中,也应该无创地跟踪干细胞的细胞分化动态以确保安全。正电子发射断层扫描 (PET) 提供分子功能信息,有助于检测特定的疾病状况。目前的研究使用 18 种方法,对免疫缺陷(非肥胖糖尿病-严重联合免疫缺陷)小鼠的 hiPSC-NS/PC 移植后中枢神经系统中的 Nestin(一种 NS/PC 标记物)阳性未分化神经细胞进行可视化。 kDa 易位蛋白 (TSPO) 配体作为标记。最近发现TSPO在啮齿动物NS/PCs中表达,并且其表达随着神经元分化的进展而减少。我们假设 TSPO 也存在于 hiPSC-NS/PC 中,并在移植后残留的未成熟神经细胞中强烈表达。结果显示,未成熟的 hiPSC-NS/PC 衍生细胞中 TSPO 表达水平较高,并且随着体外神经分化的进展,TSPO 表达降低。此外,使用 [ 18 F] FEDAC(一种 TSPO 放射性配体)进行 PET 检测能够在体内观察到由 TSPO 和 Nestin +细胞组成的剩余未分化 hiPSC-NS/PC 衍生细胞。这些发现表明,具有[ 18 F] FEDAC的 PET可以在再生医学中中枢神经系统修复的安全临床应用中发挥关键作用。

























 京公网安备 11010802027423号
京公网安备 11010802027423号