Electrocatalysis ( IF 3.1 ) Pub Date : 2019-06-08 , DOI: 10.1007/s12678-019-00539-0 José R. N. Santos , Deracilde S. S. Viégas , Ismael Carlos B. Alves , Alex D. Rabelo , Wendell M. Costa , Edmar P. Marques , Lei Zhang , Jiujun Zhang , Aldaléa L. B. Marques
The reduced graphene oxide (rGO) is used to support nickel(II)-bis(1,10-phenanthroline) complex (Ni(II)(Phen)2), forming a catalyst Ni(II)(Phen)2/rGO for ethanol oxidation reaction (EOR). A pyrolytic graphite electrode modified by this catalyst shows excellent electrocatalytic EOR activity, characterized by physical and electrochemical methods. The electrocatalytic activity of the material was evaluated by cyclic voltammetry, chronoamperometry, and electrochemical impedance spectroscopy (EIS). The significant increase in EOR currents compared to the electrode modified with only (Ni(II)(Phen)2 complex demonstrates the promotion role of the rGO. It is believed that the interaction between Ni(II)(Phen)2 and rGO to create a synergistic effect of Ni(II)(Phen)2/rGO catalyst should be responsible for the observed enhancement of the catalytic EOR performance. Using the Laviron theory, the charge transfer rate constant (ks) and the electron transfer coefficient (α) of the electrode reaction are calculated to be 0.60 s−1 and 0.61, respectively. Both the effects of OH− and ethanol concentration on the catalyst EOR activity are also studied to obtain the diffusion coefficient of ethanol (D = 4.7 × 10−6 cm2 s−1) and the catalytic rate constant (kcat = 1.26 × 107 cm3 mol−1 s−1). Based on the experimental results, an EOR mechanism catalyzed by Ni(II)(Phen)2/rGO is proposed. The catalytic EOR peak currents exhibit a linear growth (behavior) with increasing ethanol concentration, suggesting the possible use of this catalyst material as a sensor for ethanol analysis. In addition, the obtained chronoamperometric curves confirm the stability of the catalyst. It is believed that this Ni(II)(Phen)2/rGO catalyst is a promising cost-effective alternative for ethanol oxidation reaction in direct ethanol fuel cells.
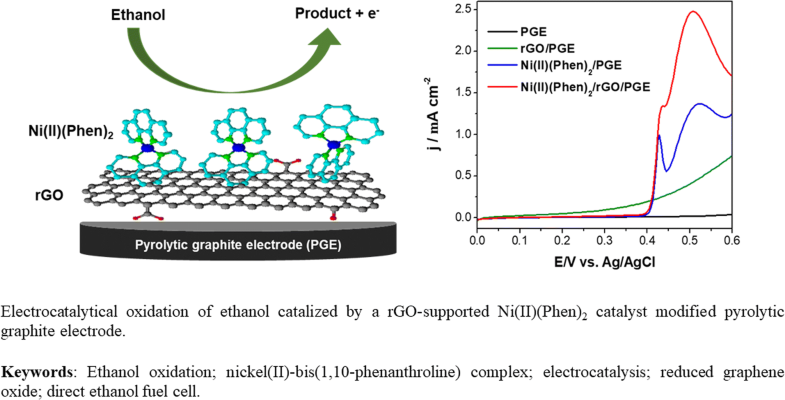
Electrocatalytical oxidation of ethanol catalyzed by a rGO-supported Ni(II)(Phen)2 catalyst-modified pyrolytic graphite electrode
中文翻译:

还原氧化石墨烯负载的镍(II)-双(1,10-菲咯啉)配合物作为乙醇氧化反应的高活性电催化剂
还原的氧化石墨烯(rGO)用于负载镍(II)-双(1,10-菲咯啉)络合物(Ni(II)(Phen)2),形成催化剂Ni(II)(Phen)2 / rGO用于乙醇氧化反应(EOR)。用该催化剂改性的热解石墨电极表现出优异的电催化EOR活性,其特征在于物理和电化学方法。通过循环伏安法,计时电流法和电化学阻抗谱(EIS)评估了材料的电催化活性。与仅用(Ni(II)(Phen)2配合物修饰的电极相比,EOR电流显着增加证明了rGO的促进作用。据信Ni(II)(Phen)2之间的相互作用rGO产生Ni(II)(Phen)2 / rGO催化剂的协同效应,应负责观察到的催化EOR性能的提高。使用拉维龙理论,电极反应的电荷转移速率常数(k s)和电子转移系数(α)分别计算为0.60 s -1和0.61。两个OH的效果-在催化剂上EOR活性和乙醇浓度进行了研究,得到的乙醇的扩散系数(d = 4.7×10 -6 厘米2 小号-1)和催化速率常数(ķ猫 = 1.26×107 cm 3 mol -1 s -1)。基于实验结果,提出了Ni(II)(Phen)2 / rGO催化的EOR机理。催化EOR峰值电流随乙醇浓度的增加而呈现线性增长(行为),这表明该催化剂材料可能用作乙醇分析的传感器。另外,所获得的计时电流曲线证实了催化剂的稳定性。据信,这种Ni(II)(Phen)2 / rGO催化剂是直接乙醇燃料电池中乙醇氧化反应的有希望的经济有效的替代品。

rGO负载的Ni(II)(Phen)2催化剂修饰的热解石墨电极对乙醇的电催化氧化


























 京公网安备 11010802027423号
京公网安备 11010802027423号