当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Two-Step Process for the Synthesis of Dimethyldichlorosilane Using Copper Aluminate Catalysts
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2020-02-17 , DOI: 10.1021/acs.iecr.9b06700 Krishna Janmanchi 1 , Aaron Coppernoll 1 , Dimitris Katsoulis 1
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2020-02-17 , DOI: 10.1021/acs.iecr.9b06700 Krishna Janmanchi 1 , Aaron Coppernoll 1 , Dimitris Katsoulis 1
Affiliation
|
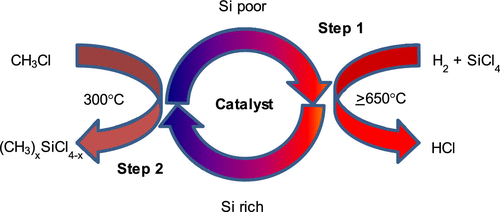
The present study relates to the development of a new generation of copper aluminate-type spinel catalysts for the production of dimethyldichlorosilane ((CH3)2SiCl2) from silicon tetrachloride (SiCl4) in a two-step reaction process. The first step is the reaction of SiCl4 with H2 over the spinel catalysts at high temperature (∼650 °C) to produce a copper–silicon-rich solid (copper silicide) by silicon deposition. In the second step, the silicon component of this solid reacts with CH3Cl at 300 °C to form (CH3)2SiCl2. Copper aluminates (CuAl2O4) were found to exhibit superior activity over other conventional copper catalysts and held better particle integrity in laboratory-scale fixed bed and fluid bed reactors. Their activity was maintained even at a 240 °C reaction temperature with CH3Cl, producing high selectivity toward (CH3)2SiCl2. Maximum production of (CH3)2SiCl2 was achieved right from the beginning of the reaction with CH3Cl without requiring any “activation period”. In contrast, the conventional Cu-supported catalysts required “an activation period” of several initial cycles to obtain maximum steady state activity. The high rate of methylchlorosilanes production on spinel catalysts is attributed to the smaller particle sizes of copper and the in situ formation of a reactive copper silicide (Cu3.17Si) phase as a result of the reaction with SiCl4/H2. The copper aluminate catalyst was tested in 45 experiments in time on stream and showed stable selectivity toward (CH3)2SiCl2 in a laboratory-scale fixed bed reactor. It demonstrated superior and acceptable attrition resistance and copper retention, whereas conventional supported copper catalysts did not retain the copper during piloting in a fluidized process. Overall, we developed a low cost, efficient, and scalable process to produce dimethyldichlorosilane ((CH3)2SiCl2).
中文翻译:

铝酸铜催化两步法合成二甲基二氯硅烷
本研究涉及新一代的铝酸铜型尖晶石催化剂的开发,该催化剂可在四步反应过程中由四氯化硅(SiCl 4)生产二甲基二氯硅烷((CH 3)2 SiCl 2)。第一步是使SiCl 4与H 2在尖晶石催化剂上于高温(约650°C)下反应,通过硅沉积生成富含铜-硅的固体(铜硅化物)。在第二步骤中,该固体的硅组分在300°C下与CH 3 Cl反应形成(CH 3)2 SiCl 2。铝酸铜(CuAl 2 O 4被发现具有比其他常规铜催化剂更高的活性,并在实验室规模的固定床和流化床反应器中保持了更好的颗粒完整性。甚至在与CH 3 Cl的反应温度为240°C时,它们的活性也得以保持,从而对(CH 3)2 SiCl 2产生了高选择性。从与CH 3反应开始就立即达到(CH 3)2 SiCl 2的最大产量。C1,不需要任何“激活期”。相反,常规的铜负载的催化剂需要几个初始循环的“活化期”以获得最大的稳态活性。尖晶石催化剂上甲基氯硅烷的高产率归因于铜的较小尺寸和与SiCl 4 / H 2反应的结果,反应性硅化铜(Cu 3.17 Si)相就地形成。铝酸铜催化剂经过45项实时实验测试,对(CH 3)2 SiCl 2具有稳定的选择性在实验室规模的固定床反应器中。它表现出优异的和可接受的耐磨损性和铜保持力,而常规的负载型铜催化剂在流化过程中的中试过程中并未保留铜。总体而言,我们开发了一种低成本,高效且可扩展的工艺来生产二甲基二氯硅烷((CH 3)2 SiCl 2)。
更新日期:2020-02-17
中文翻译:

铝酸铜催化两步法合成二甲基二氯硅烷
本研究涉及新一代的铝酸铜型尖晶石催化剂的开发,该催化剂可在四步反应过程中由四氯化硅(SiCl 4)生产二甲基二氯硅烷((CH 3)2 SiCl 2)。第一步是使SiCl 4与H 2在尖晶石催化剂上于高温(约650°C)下反应,通过硅沉积生成富含铜-硅的固体(铜硅化物)。在第二步骤中,该固体的硅组分在300°C下与CH 3 Cl反应形成(CH 3)2 SiCl 2。铝酸铜(CuAl 2 O 4被发现具有比其他常规铜催化剂更高的活性,并在实验室规模的固定床和流化床反应器中保持了更好的颗粒完整性。甚至在与CH 3 Cl的反应温度为240°C时,它们的活性也得以保持,从而对(CH 3)2 SiCl 2产生了高选择性。从与CH 3反应开始就立即达到(CH 3)2 SiCl 2的最大产量。C1,不需要任何“激活期”。相反,常规的铜负载的催化剂需要几个初始循环的“活化期”以获得最大的稳态活性。尖晶石催化剂上甲基氯硅烷的高产率归因于铜的较小尺寸和与SiCl 4 / H 2反应的结果,反应性硅化铜(Cu 3.17 Si)相就地形成。铝酸铜催化剂经过45项实时实验测试,对(CH 3)2 SiCl 2具有稳定的选择性在实验室规模的固定床反应器中。它表现出优异的和可接受的耐磨损性和铜保持力,而常规的负载型铜催化剂在流化过程中的中试过程中并未保留铜。总体而言,我们开发了一种低成本,高效且可扩展的工艺来生产二甲基二氯硅烷((CH 3)2 SiCl 2)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号