当前位置:
X-MOL 学术
›
Phytochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Metabolic fingerprinting of banana passion fruits and its correlation with quorum quenching activity
Phytochemistry ( IF 3.8 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.phytochem.2020.112272 Leonardo Castellanos 1 , Sandra Judith Naranjo-Gaybor 2 , Abel M Forero 3 , Gustavo Morales 3 , Erica Georgina Wilson 4 , Freddy A Ramos 3 , Young Hae Choi 5
Phytochemistry ( IF 3.8 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.phytochem.2020.112272 Leonardo Castellanos 1 , Sandra Judith Naranjo-Gaybor 2 , Abel M Forero 3 , Gustavo Morales 3 , Erica Georgina Wilson 4 , Freddy A Ramos 3 , Young Hae Choi 5
Affiliation
|
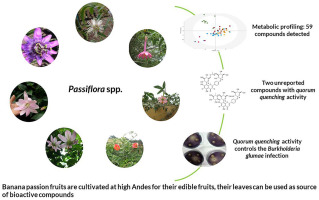
Banana passion fruit of the Passiflora genus, are commercially cultivated on a small to medium scale, mainly as edible fruits or as components of traditional herbal medicines. This subgenus comprises several species and hybrid specimens that grow readily in the wild. Due to their taxonomical complexity, many of these species have recently been reclassified (Ocampo Pérez and Coppens d'Eeckenbrugge, 2017), and their chemical profile has still to be determined. In this study, an 1H NMR-based platform was applied to the chemical profiling of seven wild species of the Passiflora subgenus, and UHPLC-DAD-MS was additionally used for the identification of phenolic compounds. A total of 59 compounds were detected including 26 O- and C-glycosidated flavonoids and polyphenols, nine organic acids, seven amino acids, GABA, sucrose, glucose, myo-inositol, and five other non-identified compounds. Two of the identified compounds are the previously undescribed C-glycosyl flavonoids, apigenin-4'-O-β-glucopyranosyl, 8-C-β-(6″acetyl)-glucopyranoside and apigenin-4-O-β-glucopyranosyl-8-C-β-neohesperidoside. These C-glycosyl flavonoids were isolated to confirm their proposed structures by NMR and LCMS analysis. The PCA score plots obtained from the 1H NMR data of the studied Passiflora samples showed P. cumbalensis and P. uribei as the species with the most distinguishable chemical profile. In addition, a correlation analysis using OPLS-DA was conducted between 1H-NMR data and the quorum quenching activity (QQ) of Chromobacterium violaceum ATCC 31532. This analysis revealed P. lehmannii, and P. uribei extracts to be the most active, and apigenin-4'-O-β-glucopyranosyl, 8-C-β-(6″acetyl)-glucopyranoside and apigenin-4-O-β-glucopyranosyl-8-C-β-neohesperidoside were identified as possibly responsible for the QQ activity. To confirm this, QQ activity of both compounds was tested against C. violaceum ATCC 3153. An inhibition of violacein production of 0.135 mM (100 μg/mL) and 0.472 mM (300 μg/mL) was observed for apigenin-4'-O-β-glucopyranosyl,8-C-β-(6″acetyl)-glucopyranoside and apigenin-4-O-β-glucopyranosyl-8-C-β-neohesperidoside respectively, while bacterial growth was unaffected in both cases. Furthermore, both compounds showed the ability to inhibit the production of the toxoflavin of the phytopathogen Burkholderia glumae ATCC 33617.
中文翻译:

香蕉百香果代谢指纹图谱及其与群体淬灭活性的相关性
西番莲属的香蕉百香果在中小规模商业化种植,主要作为可食用水果或作为传统草药的成分。该亚属包括在野外很容易生长的几个物种和杂交标本。由于它们的分类复杂性,其中许多物种最近被重新分类(Ocampo Pérez 和 Coppens d'Eeckenbrugge,2017),它们的化学特征仍有待确定。在本研究中,基于 1H NMR 的平台被应用于西番莲亚属的 7 个野生物种的化学分析,另外还使用 UHPLC-DAD-MS 来鉴定酚类化合物。共检测到 59 种化合物,包括 26 种 O-和 C-糖苷化黄酮和多酚、9 种有机酸、7 种氨基酸、GABA、蔗糖、葡萄糖、肌醇、和其他五种未鉴定的化合物。两种已鉴定的化合物是以前未描述的 C-糖基黄酮类化合物,apigenin-4'-O-β-glupyranosyl、8-C-β-(6″acetyl)-glucopyranoside 和 apigenin-4-O-β-glupyranosyl-8 -C-β-新橙皮苷。分离这些 C-糖基黄酮以通过 NMR 和 LCMS 分析确认它们提出的结构。从所研究的西番莲样品的 1H NMR 数据中获得的 PCA 得分图显示 P. cumbalensis 和 P. uribei 是具有最可区分化学特征的物种。此外,使用 OPLS-DA 进行了 1H-NMR 数据与紫色色杆菌 ATCC 31532 的群体淬灭活性 (QQ) 之间的相关性分析。该分析显示莱曼假单胞菌和乌里贝假单胞菌提取物的活性最高,并且芹菜素-4'-O-β-吡喃葡萄糖基,8-C-β-(6″乙酰基)-吡喃葡萄糖苷和芹菜素-4-O-β-吡喃葡萄糖基-8-C-β-新橙皮苷被鉴定为可能是QQ活性的原因。为了证实这一点,针对 C. violaceum ATCC 3153 测试了两种化合物的 QQ 活性。观察到 apigenin-4'-O 对紫罗兰素产生的抑制作用为 0.135 mM (100 μg/mL) 和 0.472 mM (300 μg/mL) -β-吡喃葡萄糖基、8-C-β-(6″乙酰基)-吡喃葡萄糖苷和芹菜素-4-O-β-吡喃葡萄糖基-8-C-β-新橙皮苷,而两种情况下细菌生长均未受影响。此外,这两种化合物都显示出抑制植物病原体 Burkholderia glumae ATCC 33617 的毒素黄素产生的能力。测试了两种化合物对 C. violaceum ATCC 3153 的 QQ 活性。观察到 apigenin-4'-O-β-吡喃葡萄糖基对紫罗兰素产生的抑制作用分别为 0.135 mM (100 μg/mL) 和 0.472 mM (300 μg/mL) ,8-C-β-(6″乙酰基)-吡喃葡萄糖苷和芹菜素-4-O-β-吡喃葡萄糖基-8-C-β-新橙皮苷,而两种情况下细菌生长均不受影响。此外,这两种化合物都显示出抑制植物病原体 Burkholderia glumae ATCC 33617 的毒素黄素产生的能力。测试了两种化合物对 C. violaceum ATCC 3153 的 QQ 活性。观察到 apigenin-4'-O-β-吡喃葡萄糖基对紫罗兰素产生的抑制作用分别为 0.135 mM (100 μg/mL) 和 0.472 mM (300 μg/mL) ,8-C-β-(6″乙酰基)-吡喃葡萄糖苷和芹菜素-4-O-β-吡喃葡萄糖基-8-C-β-新橙皮苷,而两种情况下细菌生长均不受影响。此外,这两种化合物都显示出抑制植物病原体 Burkholderia glumae ATCC 33617 的毒素黄素产生的能力。
更新日期:2020-04-01
中文翻译:

香蕉百香果代谢指纹图谱及其与群体淬灭活性的相关性
西番莲属的香蕉百香果在中小规模商业化种植,主要作为可食用水果或作为传统草药的成分。该亚属包括在野外很容易生长的几个物种和杂交标本。由于它们的分类复杂性,其中许多物种最近被重新分类(Ocampo Pérez 和 Coppens d'Eeckenbrugge,2017),它们的化学特征仍有待确定。在本研究中,基于 1H NMR 的平台被应用于西番莲亚属的 7 个野生物种的化学分析,另外还使用 UHPLC-DAD-MS 来鉴定酚类化合物。共检测到 59 种化合物,包括 26 种 O-和 C-糖苷化黄酮和多酚、9 种有机酸、7 种氨基酸、GABA、蔗糖、葡萄糖、肌醇、和其他五种未鉴定的化合物。两种已鉴定的化合物是以前未描述的 C-糖基黄酮类化合物,apigenin-4'-O-β-glupyranosyl、8-C-β-(6″acetyl)-glucopyranoside 和 apigenin-4-O-β-glupyranosyl-8 -C-β-新橙皮苷。分离这些 C-糖基黄酮以通过 NMR 和 LCMS 分析确认它们提出的结构。从所研究的西番莲样品的 1H NMR 数据中获得的 PCA 得分图显示 P. cumbalensis 和 P. uribei 是具有最可区分化学特征的物种。此外,使用 OPLS-DA 进行了 1H-NMR 数据与紫色色杆菌 ATCC 31532 的群体淬灭活性 (QQ) 之间的相关性分析。该分析显示莱曼假单胞菌和乌里贝假单胞菌提取物的活性最高,并且芹菜素-4'-O-β-吡喃葡萄糖基,8-C-β-(6″乙酰基)-吡喃葡萄糖苷和芹菜素-4-O-β-吡喃葡萄糖基-8-C-β-新橙皮苷被鉴定为可能是QQ活性的原因。为了证实这一点,针对 C. violaceum ATCC 3153 测试了两种化合物的 QQ 活性。观察到 apigenin-4'-O 对紫罗兰素产生的抑制作用为 0.135 mM (100 μg/mL) 和 0.472 mM (300 μg/mL) -β-吡喃葡萄糖基、8-C-β-(6″乙酰基)-吡喃葡萄糖苷和芹菜素-4-O-β-吡喃葡萄糖基-8-C-β-新橙皮苷,而两种情况下细菌生长均未受影响。此外,这两种化合物都显示出抑制植物病原体 Burkholderia glumae ATCC 33617 的毒素黄素产生的能力。测试了两种化合物对 C. violaceum ATCC 3153 的 QQ 活性。观察到 apigenin-4'-O-β-吡喃葡萄糖基对紫罗兰素产生的抑制作用分别为 0.135 mM (100 μg/mL) 和 0.472 mM (300 μg/mL) ,8-C-β-(6″乙酰基)-吡喃葡萄糖苷和芹菜素-4-O-β-吡喃葡萄糖基-8-C-β-新橙皮苷,而两种情况下细菌生长均不受影响。此外,这两种化合物都显示出抑制植物病原体 Burkholderia glumae ATCC 33617 的毒素黄素产生的能力。测试了两种化合物对 C. violaceum ATCC 3153 的 QQ 活性。观察到 apigenin-4'-O-β-吡喃葡萄糖基对紫罗兰素产生的抑制作用分别为 0.135 mM (100 μg/mL) 和 0.472 mM (300 μg/mL) ,8-C-β-(6″乙酰基)-吡喃葡萄糖苷和芹菜素-4-O-β-吡喃葡萄糖基-8-C-β-新橙皮苷,而两种情况下细菌生长均不受影响。此外,这两种化合物都显示出抑制植物病原体 Burkholderia glumae ATCC 33617 的毒素黄素产生的能力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号