当前位置:
X-MOL 学术
›
Nat. Cell Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
HSF1 phase transition mediates stress adaptation and cell fate decisions.
Nature Cell Biology ( IF 21.3 ) Pub Date : 2020-02-03 , DOI: 10.1038/s41556-019-0458-3 Giorgio Gaglia 1, 2, 3 , Rumana Rashid 2, 3, 4 , Clarence Yapp 4, 5 , Gaurav N Joshi 6, 7 , Carmen G Li 2 , Susan L Lindquist 1, 8, 9 , Kristopher A Sarosiek 4, 6, 10 , Luke Whitesell 1, 11 , Peter K Sorger 3, 4 , Sandro Santagata 2, 3, 4, 12
Nature Cell Biology ( IF 21.3 ) Pub Date : 2020-02-03 , DOI: 10.1038/s41556-019-0458-3 Giorgio Gaglia 1, 2, 3 , Rumana Rashid 2, 3, 4 , Clarence Yapp 4, 5 , Gaurav N Joshi 6, 7 , Carmen G Li 2 , Susan L Lindquist 1, 8, 9 , Kristopher A Sarosiek 4, 6, 10 , Luke Whitesell 1, 11 , Peter K Sorger 3, 4 , Sandro Santagata 2, 3, 4, 12
Affiliation
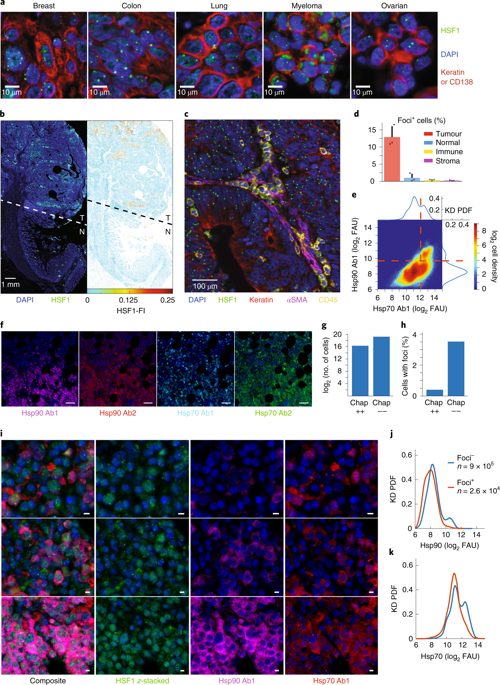
|
Under proteotoxic stress, some cells survive whereas others die. The mechanisms governing this heterogeneity in cell fate remain unknown. Here we report that condensation and phase transition of heat-shock factor 1 (HSF1), a transcriptional regulator of chaperones1,2, is integral to cell-fate decisions underlying survival or death. During stress, HSF1 drives chaperone expression but also accumulates separately in nuclear stress bodies called foci3-6. Foci formation has been regarded as a marker of cells actively upregulating chaperones3,6-10. Using multiplexed tissue imaging, we observed HSF1 foci in human tumours. Paradoxically, their presence inversely correlated with chaperone expression. By live-cell microscopy and single-cell analysis, we found that foci dissolution rather than formation promoted HSF1 activity and cell survival. During prolonged stress, the biophysical properties of HSF1 foci changed; small, fluid condensates enlarged into indissoluble gel-like arrangements with immobilized HSF1. Chaperone gene induction was reduced in such cells, which were prone to apoptosis. Quantitative analysis suggests that survival under stress results from competition between concurrent but opposing mechanisms. Foci may serve as sensors that tune cytoprotective responses, balancing rapid transient responses and irreversible outcomes.
中文翻译:

HSF1 相变介导压力适应和细胞命运决定。
在蛋白质毒性压力下,一些细胞存活,而另一些则死亡。控制这种细胞命运异质性的机制仍然未知。在这里,我们报告热休克因子 1 (HSF1) 的凝聚和相变,一种伴侣蛋白 1,2 的转录调节因子,是细胞命运决定生存或死亡的组成部分。在压力期间,HSF1 驱动伴侣表达,但也分别在称为 foci3-6 的核压力体中积累。病灶形成被认为是细胞主动上调伴侣蛋白3,6-10 的标志物。使用多重组织成像,我们观察到人类肿瘤中的 HSF1 病灶。矛盾的是,它们的存在与伴侣的表达呈负相关。通过活细胞显微镜和单细胞分析,我们发现病灶溶解而不是形成促进了 HSF1 活性和细胞存活。在长时间的压力下,HSF1 病灶的生物物理特性发生了变化;小的,流体冷凝物扩大成不可溶解的凝胶状排列,并带有固定的 HSF1。在这些细胞中,伴侣基因诱导减少,容易发生凋亡。定量分析表明,压力下的生存是由同时但相反的机制之间的竞争造成的。Foci 可以作为调节细胞保护反应、平衡快速瞬态反应和不可逆结果的传感器。定量分析表明,压力下的生存是由同时但相反的机制之间的竞争造成的。Foci 可以作为调节细胞保护反应、平衡快速瞬态反应和不可逆结果的传感器。定量分析表明,压力下的生存是由同时但相反的机制之间的竞争造成的。Foci 可以作为调节细胞保护反应、平衡快速瞬态反应和不可逆结果的传感器。
更新日期:2020-02-03
中文翻译:

HSF1 相变介导压力适应和细胞命运决定。
在蛋白质毒性压力下,一些细胞存活,而另一些则死亡。控制这种细胞命运异质性的机制仍然未知。在这里,我们报告热休克因子 1 (HSF1) 的凝聚和相变,一种伴侣蛋白 1,2 的转录调节因子,是细胞命运决定生存或死亡的组成部分。在压力期间,HSF1 驱动伴侣表达,但也分别在称为 foci3-6 的核压力体中积累。病灶形成被认为是细胞主动上调伴侣蛋白3,6-10 的标志物。使用多重组织成像,我们观察到人类肿瘤中的 HSF1 病灶。矛盾的是,它们的存在与伴侣的表达呈负相关。通过活细胞显微镜和单细胞分析,我们发现病灶溶解而不是形成促进了 HSF1 活性和细胞存活。在长时间的压力下,HSF1 病灶的生物物理特性发生了变化;小的,流体冷凝物扩大成不可溶解的凝胶状排列,并带有固定的 HSF1。在这些细胞中,伴侣基因诱导减少,容易发生凋亡。定量分析表明,压力下的生存是由同时但相反的机制之间的竞争造成的。Foci 可以作为调节细胞保护反应、平衡快速瞬态反应和不可逆结果的传感器。定量分析表明,压力下的生存是由同时但相反的机制之间的竞争造成的。Foci 可以作为调节细胞保护反应、平衡快速瞬态反应和不可逆结果的传感器。定量分析表明,压力下的生存是由同时但相反的机制之间的竞争造成的。Foci 可以作为调节细胞保护反应、平衡快速瞬态反应和不可逆结果的传感器。


























 京公网安备 11010802027423号
京公网安备 11010802027423号