当前位置:
X-MOL 学术
›
Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A molecular level insight into adsorption of β-lactam antibiotics on vermiculite surface
Surface Science ( IF 1.9 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.susc.2020.121588 Nguyen Ngoc Tri , Minh Tho Nguyen , Nguyen Tien Trung
Surface Science ( IF 1.9 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.susc.2020.121588 Nguyen Ngoc Tri , Minh Tho Nguyen , Nguyen Tien Trung
|
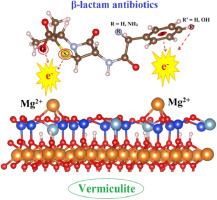
Abstract The adsorption processes of β-lactam antibiotics including ampicillin (AP), amoxicillin (AX) and benzylpenicillin (BP) on the vermiculite surface were investigated using density functional theory computations. The C09-vdW density functional was used to include van der Waals interactions for determination of the most stable configurations governing the interactions between AP, AX and BP molecules and the vermiculite surface. Each molecule prefers to arrange horizontally on the surface to form both Mg∙∙∙S and Mg∙∙∙π contacts, or two Mg∙∙∙O electrostatic interactions between S atom in CS, π-electrons of benzene ring or O atoms of -COOH, OH groups in molecules and Mg2+ sites on surface. Remarkably, an important role of Mg∙∙∙π interaction in the complex stabilization has been observed for the first time. These are strong chemisorption processes with adsorption energies ranging from −72 to −78 kcal.mol−1. AIM calculations show a significant contribution of Mg∙∙∙O/S/π interactions and O H∙∙∙O hydrogen bonds to the complex stabilization. Formation and role of different interactions on the stability of complexes were thoroughly examined by MOs and EDTs analysis based on NBO approach. Overall, vermiculite, a clay mineral, emerges to offer an efficient adsorption surface and can be used as a material to remove antibiotic molecules from waste water sources.
中文翻译:

β-内酰胺类抗生素吸附在蛭石表面的分子水平洞察
摘要 采用密度泛函理论计算研究了氨苄青霉素(AP)、阿莫西林(AX)和苄青霉素(BP)等β-内酰胺类抗生素在蛭石表面的吸附过程。C09-vdW 密度泛函用于包括范德华相互作用,以确定控制 AP、AX 和 BP 分子与蛭石表面之间相互作用的最稳定构型。每个分子更喜欢在表面水平排列,形成 Mg∙∙∙S 和 Mg∙∙∙π 接触,或在 CS 中的 S 原子、苯环的 π 电子或 O 原子之间的两个 Mg∙∙∙O 静电相互作用分子中的 -COOH、OH 基团和表面的 Mg2+ 位点。值得注意的是,首次观察到 Mg∙∙∙π 相互作用在复合物稳定中的重要作用。这些是强化学吸附过程,吸附能范围从 -72 到 -78 kcal.mol-1。AIM 计算表明 Mg∙∙∙O/S/π 相互作用和 OH∙∙∙O 氢键对复合物的稳定性有显着贡献。基于 NBO 方法的 MOs 和 EDTs 分析彻底检查了不同相互作用对配合物稳定性的形成和作用。总体而言,蛭石是一种粘土矿物,可提供有效的吸附表面,并可用作从废水源中去除抗生素分子的材料。基于 NBO 方法的 MOs 和 EDTs 分析彻底检查了不同相互作用对配合物稳定性的形成和作用。总体而言,蛭石是一种粘土矿物,可提供有效的吸附表面,并可用作从废水源中去除抗生素分子的材料。基于 NBO 方法的 MOs 和 EDTs 分析彻底检查了不同相互作用对配合物稳定性的形成和作用。总体而言,蛭石是一种粘土矿物,可提供有效的吸附表面,并可用作从废水源中去除抗生素分子的材料。
更新日期:2020-05-01
中文翻译:

β-内酰胺类抗生素吸附在蛭石表面的分子水平洞察
摘要 采用密度泛函理论计算研究了氨苄青霉素(AP)、阿莫西林(AX)和苄青霉素(BP)等β-内酰胺类抗生素在蛭石表面的吸附过程。C09-vdW 密度泛函用于包括范德华相互作用,以确定控制 AP、AX 和 BP 分子与蛭石表面之间相互作用的最稳定构型。每个分子更喜欢在表面水平排列,形成 Mg∙∙∙S 和 Mg∙∙∙π 接触,或在 CS 中的 S 原子、苯环的 π 电子或 O 原子之间的两个 Mg∙∙∙O 静电相互作用分子中的 -COOH、OH 基团和表面的 Mg2+ 位点。值得注意的是,首次观察到 Mg∙∙∙π 相互作用在复合物稳定中的重要作用。这些是强化学吸附过程,吸附能范围从 -72 到 -78 kcal.mol-1。AIM 计算表明 Mg∙∙∙O/S/π 相互作用和 OH∙∙∙O 氢键对复合物的稳定性有显着贡献。基于 NBO 方法的 MOs 和 EDTs 分析彻底检查了不同相互作用对配合物稳定性的形成和作用。总体而言,蛭石是一种粘土矿物,可提供有效的吸附表面,并可用作从废水源中去除抗生素分子的材料。基于 NBO 方法的 MOs 和 EDTs 分析彻底检查了不同相互作用对配合物稳定性的形成和作用。总体而言,蛭石是一种粘土矿物,可提供有效的吸附表面,并可用作从废水源中去除抗生素分子的材料。基于 NBO 方法的 MOs 和 EDTs 分析彻底检查了不同相互作用对配合物稳定性的形成和作用。总体而言,蛭石是一种粘土矿物,可提供有效的吸附表面,并可用作从废水源中去除抗生素分子的材料。



























 京公网安备 11010802027423号
京公网安备 11010802027423号