当前位置:
X-MOL 学术
›
Nat. Struct. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Atomic structures of closed and open influenza B M2 proton channel reveal the conduction mechanism.
Nature Structural & Molecular Biology ( IF 16.8 ) Pub Date : 2020-02-03 , DOI: 10.1038/s41594-019-0371-2 Venkata S Mandala 1 , Alexander R Loftis 1 , Alexander A Shcherbakov 1 , Bradley L Pentelute 1 , Mei Hong 1
Nature Structural & Molecular Biology ( IF 16.8 ) Pub Date : 2020-02-03 , DOI: 10.1038/s41594-019-0371-2 Venkata S Mandala 1 , Alexander R Loftis 1 , Alexander A Shcherbakov 1 , Bradley L Pentelute 1 , Mei Hong 1
Affiliation
|
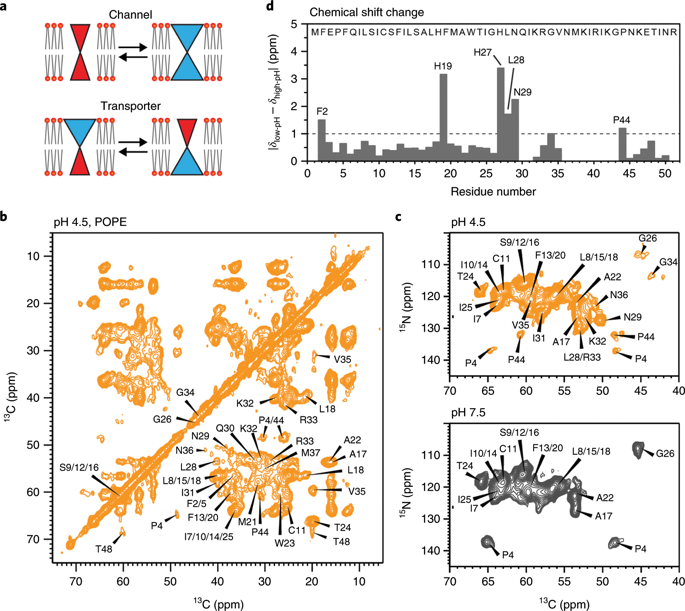
The influenza B M2 (BM2) proton channel is activated by acidic pH to mediate virus uncoating. Unlike influenza A M2 (AM2), which conducts protons with strong inward rectification, BM2 conducts protons both inward and outward. Here we report 1.4- and 1.5-Å solid-state NMR structures of the transmembrane domain of the closed and open BM2 channels in a phospholipid environment. Upon activation, the transmembrane helices increase the tilt angle by 6° and the average pore diameter enlarges by 2.1 Å. BM2 thus undergoes a scissor motion for activation, which differs from the alternating-access motion of AM2. These results indicate that asymmetric proton conduction requires a backbone hinge motion, whereas bidirectional conduction is achieved by a symmetric scissor motion. The proton-selective histidine and gating tryptophan in the open BM2 reorient on the microsecond timescale, similar to AM2, indicating that side chain dynamics are the essential driver of proton shuttling.
中文翻译:

封闭和开放的乙型流感 M2 质子通道的原子结构揭示了传导机制。
乙型流感 M2 (BM2) 质子通道被酸性 pH 激活以介导病毒脱壳。与甲型流感 M2 (AM2) 传导质子并具有强烈的内向整流不同,BM2 向内和向外传导质子。在这里,我们报告了磷脂环境中闭合和开放 BM2 通道的跨膜域的 1.4 和 1.5 Å 固态 NMR 结构。激活后,跨膜螺旋将倾斜角增加 6°,平均孔径扩大 2.1 Å。因此,BM2 经历了一个剪刀运动来激活,这与 AM2 的交替访问运动不同。这些结果表明不对称质子传导需要骨干铰链运动,而双向传导是通过对称剪刀运动实现的。
更新日期:2020-02-03
中文翻译:

封闭和开放的乙型流感 M2 质子通道的原子结构揭示了传导机制。
乙型流感 M2 (BM2) 质子通道被酸性 pH 激活以介导病毒脱壳。与甲型流感 M2 (AM2) 传导质子并具有强烈的内向整流不同,BM2 向内和向外传导质子。在这里,我们报告了磷脂环境中闭合和开放 BM2 通道的跨膜域的 1.4 和 1.5 Å 固态 NMR 结构。激活后,跨膜螺旋将倾斜角增加 6°,平均孔径扩大 2.1 Å。因此,BM2 经历了一个剪刀运动来激活,这与 AM2 的交替访问运动不同。这些结果表明不对称质子传导需要骨干铰链运动,而双向传导是通过对称剪刀运动实现的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号