当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Struvite crystallization by using raw seawater: Improving economics and environmental footprint while maintaining phosphorus recovery and product quality.
Water Research ( IF 12.8 ) Pub Date : 2020-01-31 , DOI: 10.1016/j.watres.2020.115572 Sina Shaddel 1 , Tonje Grini 1 , Seniz Ucar 2 , Kamal Azrague 3 , Jens-Petter Andreassen 2 , Stein W Østerhus 1
Water Research ( IF 12.8 ) Pub Date : 2020-01-31 , DOI: 10.1016/j.watres.2020.115572 Sina Shaddel 1 , Tonje Grini 1 , Seniz Ucar 2 , Kamal Azrague 3 , Jens-Petter Andreassen 2 , Stein W Østerhus 1
Affiliation
|
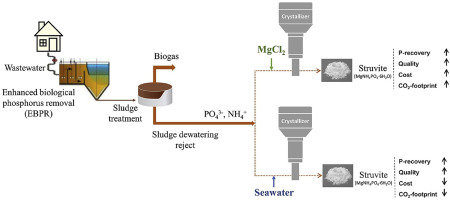
Seawater, as an alternative magnesium source, has the potential to improve the overall economics and environmental footprint of struvite production compared to the use of pure magnesium salts. However, the dilution effect and the presence of other ions in seawater can reduce the phosphorus recovery potential and the simultaneous precipitation of other compounds may reduce the quality of the produced struvite. This work presents a comparative study of seawater and MgCl2 by performing a series of thermodynamic equilibrium modeling and crystallization experiments. The results revealed that acceptable phosphorus recovery (80-90%) is achievable by using seawater as the magnesium source for struvite precipitation. Further, the simultaneous precipitation of calcium phosphates was successfully controlled and minimized by optimum selection of reaction pH and seawater volume (i.e. Mg:P and Mg:Ca molar ratios). The increase of temperature from 20 °C to 30 °C reduced the phosphorus recovery by 15-20% while it increased the particle size by 30-35%. The presence of suspended solids in reject water did not have significant effects on phosphorus recovery but it made the struvite separation difficult as the obtained struvite was mixed with suspended solids. The experimental results and economic evaluation showed that the use of seawater can reduce the chemical costs (30-50%) and the CO2-footprint (8-40%) of struvite production. It was concluded that seawater is a potential alternative to pure magnesium sources in struvite production, while studies in larger scale and continuous mode are needed for further verification before full-scale applications.
中文翻译:

使用原海水使鸟粪石结晶:在保持磷回收率和产品质量的同时,提高经济效益和环境足迹。
与使用纯镁盐相比,海水作为镁的替代来源具有改善鸟粪石生产的总体经济和环境足迹的潜力。但是,稀释作用和海水中其他离子的存在会降低磷的回收潜力,同时其他化合物的沉淀会降低所生产鸟粪石的质量。这项工作通过执行一系列热力学平衡模型和结晶实验,对海水和MgCl2进行了比较研究。结果表明,通过使用海水作为鸟粪石沉淀的镁源,可以实现可接受的磷回收率(80-90%)。进一步,通过最佳选择反应pH和海水量(即Mg:P和Mg:Ca摩尔比),成功地控制了磷酸钙的同时沉淀并使其最小化。温度从20°C升高到30°C,磷回收率降低了15-20%,而粒径增加了30-35%。废水中悬浮固体的存在对磷的回收率没有显着影响,但由于将鸟粪石与悬浮固体混合,因此鸟粪石的分离变得困难。实验结果和经济评估表明,使用海水可以减少鸟粪石生产的化学成本(30-50%)和二氧化碳排放量(8-40%)。结论是,在鸟粪石生产中,海水是纯镁来源的潜在替代品,
更新日期:2020-01-31
中文翻译:

使用原海水使鸟粪石结晶:在保持磷回收率和产品质量的同时,提高经济效益和环境足迹。
与使用纯镁盐相比,海水作为镁的替代来源具有改善鸟粪石生产的总体经济和环境足迹的潜力。但是,稀释作用和海水中其他离子的存在会降低磷的回收潜力,同时其他化合物的沉淀会降低所生产鸟粪石的质量。这项工作通过执行一系列热力学平衡模型和结晶实验,对海水和MgCl2进行了比较研究。结果表明,通过使用海水作为鸟粪石沉淀的镁源,可以实现可接受的磷回收率(80-90%)。进一步,通过最佳选择反应pH和海水量(即Mg:P和Mg:Ca摩尔比),成功地控制了磷酸钙的同时沉淀并使其最小化。温度从20°C升高到30°C,磷回收率降低了15-20%,而粒径增加了30-35%。废水中悬浮固体的存在对磷的回收率没有显着影响,但由于将鸟粪石与悬浮固体混合,因此鸟粪石的分离变得困难。实验结果和经济评估表明,使用海水可以减少鸟粪石生产的化学成本(30-50%)和二氧化碳排放量(8-40%)。结论是,在鸟粪石生产中,海水是纯镁来源的潜在替代品,


























 京公网安备 11010802027423号
京公网安备 11010802027423号